Genital Warts Market Outlook 2025-2035:
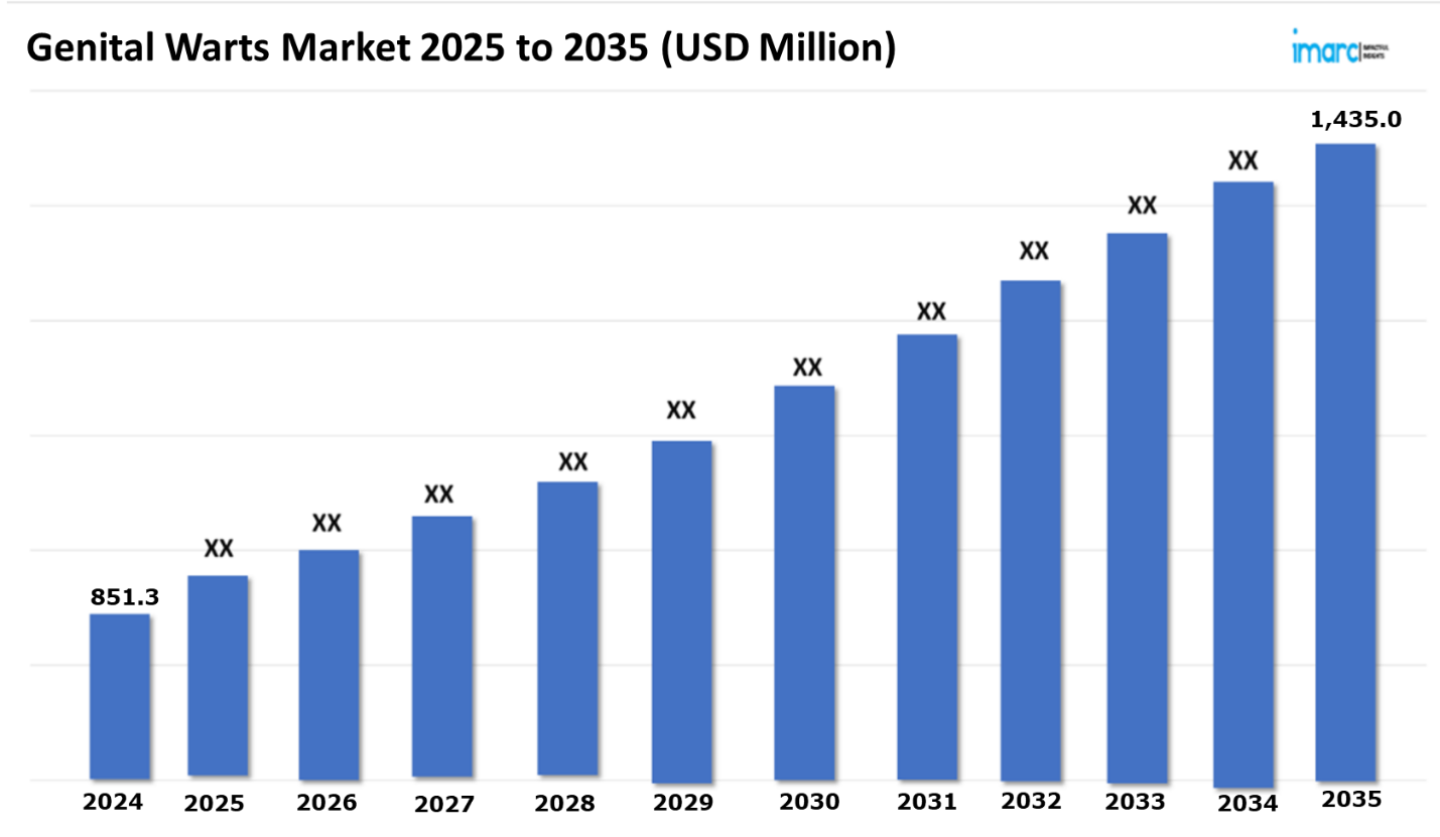
The 7 major genital warts market reached a value of USD 851.3 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 1,435.0 Million by 2035, exhibiting a growth rate (CAGR) of 4.89% during 2025-2035. The industry is principally influenced by the heightening requirement for interferon alfa-n3 injection to manage the ailment, since it can be administered directly into the lesion with a faster onset of action. In addition to this, the formulation of advanced treatments and targeted therapies is further accelerating the market expansion.
Advances in Early Detection and Diagnostic Technologies: Driving the Genital Warts Market
Advancements in early detection and diagnostic technologies are significantly driving the genital warts market by enabling timely diagnosis, improving treatment outcomes, and reducing transmission rates. Genital warts, resulting from infection of the human papillomavirus (HPV), are one of the most prominently sexually transmitted infections (STIs) globally. Early detection exhibits a pivotal role in managing the disease efficiently and mitigating complications, encompassing the progression of HPV-associated cancers. Molecular diagnostics, such as polymerase chain reaction (PCR) and HPV DNA testing, have revolutionized the early detection of genital warts. These highly sensitive techniques allow for the identification of high-risk and low-risk HPV strains, even before visible symptoms appear. This enables healthcare providers to implement timely treatment strategies and prevent further spread of the infection. Additionally, next-generation sequencing (NGS) and genotyping have enhanced HPV strain identification, allowing for more precise and personalized therapeutic approaches. Advances in imaging technologies, such as high-resolution anoscopy (HRA) and colposcopy, have improved the detection of subclinical genital warts and precancerous lesions. These techniques provide detailed visualization of affected areas, ensuring accurate diagnosis and targeted treatment interventions. The increasing availability of point-of-care testing and home-based diagnostic kits is further expanding market growth by enabling convenient and rapid detection. As these technologies continue to evolve, they contribute to the overall expansion of the genital warts market by improving early diagnosis, enhancing treatment efficacy, and reducing the burden of HPV-related diseases worldwide.
Request a PDF Sample Report: https://www.imarcgroup.com/genital-warts-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The formulation of new pharmacological treatments and novel therapies is substantially bolstering the growth of the genital warts industry by improving patient outcomes, enhancing treatment effectiveness, and lowering recurrence rates. One of the most crucial advancements in genital warts treatment is the designing of immune-modulating therapies. New systemic as well as topical immunotherapies are developed to significantly improve the body’s immune action against HPV, lowering both the recurrence and intensity of warts. For instance, innovations in therapeutic vaccines and interferon-based treatments are exhibiting successful results in elevating antiviral immunity, potentially resulting in a long-term protection against HPV-associated warts. In addition to this, the rapid rising of antiviral agents targeting HPV replication has paved new prospects for minimizing wart persistence as well as transmission. Research efforts for small-molecule inhibitors and nucleoside analogs targets to disrupt HPV’s efficiency of getting incorporated into host cells, providing a more efficient and direct tactic to mitigate genital warts condition. Besides this, , gene-editing methodologies, prominently encompassing CRISPR, are currently being explored for their high efficacy in eradicating HPV infections at a genetic level, highlighting a revolutionary step toward long-term cures. Such enhancements , coupled with heightening availability as well as awareness regarding innovative treatments, are escalating market expansion. As pharmaceutical firms are heavily investing in research and development initiatives, the launch of such novel therapies is positioned to transform genital warts management, proliferating treatment options, enhancing patient outcomes, and ultimately bolstering the growth of the genital warts industry on a global level globally.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7168&method=809
Marketed Therapies in Genital Warts Market
Aldara (Imiquimod): 3M Pharmaceuticals
Aldara (Imiquimod) is a toll-like receptor 7 (TLR7) agonist that treats genital and perianal warts. The drug functions as a modifier of immune response, triggering the innate immune system by activating TLR7, thereby prompting the production of cytokines that incentivize the body's immune cells to attack and disrupt cells infected by HPV, which results in genital warts. It generally plays role in activation of the body's natural defense mechanisms to neutralize or destroy the virus that instigates warts condition.
Veregen (Polyphenon E): Aresus Pharma/Epitome Pharmaceuticals
Veregen (Polyphenon E), developed by Aresus Pharma and Epitome Pharmaceuticals, is a topical ointment derived from green tea catechins used for treating external genital and perianal warts caused by HPV. Its mechanism of action involves multiple pathways, including antiviral, immunomodulatory, and anti-inflammatory effects. Polyphenon E inhibits HPV replication by interfering with viral protein synthesis and disrupts infected cell membranes, leading to wart regression. Additionally, it enhances the immune response by stimulating cytokine production, which helps the body recognize and clear HPV-infected cells. This multi-targeted approach makes Veregen an effective non-invasive treatment for HPV-induced genital warts.
Gardasil 9 (Human Papillomavirus 9-valent Vaccine) - Merck and Co
Gardasil 9, developed by Merck & Co., is a 9-valent human papillomavirus vaccine designed to prevent infections caused by HPV types responsible for genital warts and HPV-related cancers. Its mechanism of action involves inducing an immune response by exposing the body to virus-like particles (VLPs) that mimic HPV but lack viral DNA, making them non-infectious. This stimulates the production of neutralizing antibodies, which block HPV from infecting cells. By targeting HPV types 6 and 11, the primary causes of genital warts, Gardasil 9 effectively prevents infection, reducing the incidence of warts and associated complications.
Emerging Therapies in Genital Warts Market
SB 206: Novan
SB 206, designed by Novan, is an anti-viral topical gel that is developed to mitigate viral skin infections, including external genital and perianal warts resulting from HPV. This medication works by releasing nitric oxide (NO), which directly attacks and destroys the HPV responsible for genital warts, effectively inhibiting viral replication and potentially preventing recurrence by exerting a viricidal effect at the site of infection. Essentially, the NO released by SB 206 specifically targets and kills the virus that causes the warts.
VP-102: Verrica Pharamceuticals
VP-102, developed by Verrica Pharmaceuticals, is a topical drug-device combination containing cantharidin for the treatment of genital warts. Its mechanism of action involves inducing controlled blister formation by disrupting desmosomal cell adhesion in HPV-infected skin. Cantharidin, a vesicant, penetrates the epidermis and causes keratinocyte apoptosis, leading to the wart’s natural detachment. This targeted destruction of infected tissue helps eliminate HPV-infected cells while minimizing systemic absorption. VP-102’s precise application through its proprietary applicator ensures controlled dosing, reducing the risk of surrounding tissue damage.
Detailed list of emerging therapies in Genital Warts is provided in the final report…
Leading Companies in the Genital Warts Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global genital warts market, several leading companies are at the forefront of developing integrated platforms to enhance the management of genital warts. Some of the major players include 3M Pharmaceuticals, Merck and Co., and Aresus Pharma. These companies are driving innovation in the genital warts market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for the illness.
In September 2024, Merck publications' revealed positive top-line Phase 3 trial (V503-064) outcomes for GARDASIL 9 (Human Papillomavirus 9-valent Vaccine, Recombinant) when used as a 9-valent Human Papillomavirus vaccine in Japanese males between 16 and 26 years old. The trial successfully met both its primary and secondary objectives by showing GARDASIL 9 administered in three doses reduced the development of persistent nine-HPV strain infections in genitourinary sites versus placebo dosing.
Request for customization: https://www.imarcgroup.com/request?type=report&id=7168&flag=E
Key Players in Genital Warts Market:
The key players in the Genital Warts market who are in different phases of developing different therapies are Novan, 3M Pharmaceuticals, Aresus Pharma, Orgenesis, Epitome Pharmaceuticals, Merck and Co., G&E Herbal Biotechnology, Verrica Pharamceuticals, and Others.
Regional Analysis:
The major markets for genital warts include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for genital warts while also representing the biggest market for its treatment. This can be attributed to the rising prevalence of human papillomavirus infections, advancements in treatment options, increasing awareness and vaccination programs, and strong healthcare infrastructure.
Moreover, a major driver is the widespread adoption of HPV vaccination programs, particularly with Gardasil 9, which protects against multiple HPV strains, including those responsible for genital warts. Increased vaccine coverage has contributed to a decline in new cases, yet the need for effective treatments remains due to the millions already infected.
Besides this, pharmaceutical advancements, including topical therapies like imiquimod, sinecatechins (Veregen), and innovative treatments like VP-102 (cantharidin-based therapy), are expanding market opportunities. The rise of minimally invasive procedures such as cryotherapy and laser ablation has also improved patient outcomes, increasing demand for specialized treatments.
Recent Developments in Genital Warts Market:
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
This report offers a comprehensive analysis of current genital warts marketed drugs and late-stage pipeline drugs.
In-Market Drugs
IMARC Group Offer Other Reports:
Primary Immune Deficiency Market: The 7 major primary immune deficiency markets reached a value of US$ 5.7 Billion in 2023, and projected the 7MM to reach US$ 9.4 Billion by 2034, exhibiting a growth rate (CAGR) of 4.61% during 2024-2034.
Cytomegalovirus Infections Market: The 7 major cytomegalovirus infections markets are expected to exhibit a CAGR of 4.32% during 2024-2034.
Viral Hepatitis Market: The 7 major viral hepatitis markets are expected to exhibit a CAGR of 2.69% during 2024-2034.
Hepatitis C Market: The 7 major hepatitis c markets reached a value of US$ 18.0 Billion in 2023, and projected the 7MM to reach US$ 47.1 Billion by 2034, exhibiting a growth rate (CAGR) of 9.14% during 2024-2034.
Hepatitis D Market: The 7 major hepatitis D markets reached a value of US$ 665.1 Million in 2023, and projected the 7MM to reach US$ 845.3 Million by 2034, exhibiting a growth rate (CAGR) of 2.2% during 2024-2034.
Hepatic Encephalopathy Market: The 7 major hepatic encephalopathy markets reached a value of US$ 1.7 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 2.9 Billion by 2034, exhibiting a growth rate (CAGR) of 5.1% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The 7 major genital warts market reached a value of USD 851.3 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 1,435.0 Million by 2035, exhibiting a growth rate (CAGR) of 4.89% during 2025-2035. The industry is principally influenced by the heightening requirement for interferon alfa-n3 injection to manage the ailment, since it can be administered directly into the lesion with a faster onset of action. In addition to this, the formulation of advanced treatments and targeted therapies is further accelerating the market expansion.
Advances in Early Detection and Diagnostic Technologies: Driving the Genital Warts Market
Advancements in early detection and diagnostic technologies are significantly driving the genital warts market by enabling timely diagnosis, improving treatment outcomes, and reducing transmission rates. Genital warts, resulting from infection of the human papillomavirus (HPV), are one of the most prominently sexually transmitted infections (STIs) globally. Early detection exhibits a pivotal role in managing the disease efficiently and mitigating complications, encompassing the progression of HPV-associated cancers. Molecular diagnostics, such as polymerase chain reaction (PCR) and HPV DNA testing, have revolutionized the early detection of genital warts. These highly sensitive techniques allow for the identification of high-risk and low-risk HPV strains, even before visible symptoms appear. This enables healthcare providers to implement timely treatment strategies and prevent further spread of the infection. Additionally, next-generation sequencing (NGS) and genotyping have enhanced HPV strain identification, allowing for more precise and personalized therapeutic approaches. Advances in imaging technologies, such as high-resolution anoscopy (HRA) and colposcopy, have improved the detection of subclinical genital warts and precancerous lesions. These techniques provide detailed visualization of affected areas, ensuring accurate diagnosis and targeted treatment interventions. The increasing availability of point-of-care testing and home-based diagnostic kits is further expanding market growth by enabling convenient and rapid detection. As these technologies continue to evolve, they contribute to the overall expansion of the genital warts market by improving early diagnosis, enhancing treatment efficacy, and reducing the burden of HPV-related diseases worldwide.
Request a PDF Sample Report: https://www.imarcgroup.com/genital-warts-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The formulation of new pharmacological treatments and novel therapies is substantially bolstering the growth of the genital warts industry by improving patient outcomes, enhancing treatment effectiveness, and lowering recurrence rates. One of the most crucial advancements in genital warts treatment is the designing of immune-modulating therapies. New systemic as well as topical immunotherapies are developed to significantly improve the body’s immune action against HPV, lowering both the recurrence and intensity of warts. For instance, innovations in therapeutic vaccines and interferon-based treatments are exhibiting successful results in elevating antiviral immunity, potentially resulting in a long-term protection against HPV-associated warts. In addition to this, the rapid rising of antiviral agents targeting HPV replication has paved new prospects for minimizing wart persistence as well as transmission. Research efforts for small-molecule inhibitors and nucleoside analogs targets to disrupt HPV’s efficiency of getting incorporated into host cells, providing a more efficient and direct tactic to mitigate genital warts condition. Besides this, , gene-editing methodologies, prominently encompassing CRISPR, are currently being explored for their high efficacy in eradicating HPV infections at a genetic level, highlighting a revolutionary step toward long-term cures. Such enhancements , coupled with heightening availability as well as awareness regarding innovative treatments, are escalating market expansion. As pharmaceutical firms are heavily investing in research and development initiatives, the launch of such novel therapies is positioned to transform genital warts management, proliferating treatment options, enhancing patient outcomes, and ultimately bolstering the growth of the genital warts industry on a global level globally.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7168&method=809
Marketed Therapies in Genital Warts Market
Aldara (Imiquimod): 3M Pharmaceuticals
Aldara (Imiquimod) is a toll-like receptor 7 (TLR7) agonist that treats genital and perianal warts. The drug functions as a modifier of immune response, triggering the innate immune system by activating TLR7, thereby prompting the production of cytokines that incentivize the body's immune cells to attack and disrupt cells infected by HPV, which results in genital warts. It generally plays role in activation of the body's natural defense mechanisms to neutralize or destroy the virus that instigates warts condition.
Veregen (Polyphenon E): Aresus Pharma/Epitome Pharmaceuticals
Veregen (Polyphenon E), developed by Aresus Pharma and Epitome Pharmaceuticals, is a topical ointment derived from green tea catechins used for treating external genital and perianal warts caused by HPV. Its mechanism of action involves multiple pathways, including antiviral, immunomodulatory, and anti-inflammatory effects. Polyphenon E inhibits HPV replication by interfering with viral protein synthesis and disrupts infected cell membranes, leading to wart regression. Additionally, it enhances the immune response by stimulating cytokine production, which helps the body recognize and clear HPV-infected cells. This multi-targeted approach makes Veregen an effective non-invasive treatment for HPV-induced genital warts.
Gardasil 9 (Human Papillomavirus 9-valent Vaccine) - Merck and Co
Gardasil 9, developed by Merck & Co., is a 9-valent human papillomavirus vaccine designed to prevent infections caused by HPV types responsible for genital warts and HPV-related cancers. Its mechanism of action involves inducing an immune response by exposing the body to virus-like particles (VLPs) that mimic HPV but lack viral DNA, making them non-infectious. This stimulates the production of neutralizing antibodies, which block HPV from infecting cells. By targeting HPV types 6 and 11, the primary causes of genital warts, Gardasil 9 effectively prevents infection, reducing the incidence of warts and associated complications.
Emerging Therapies in Genital Warts Market
SB 206: Novan
SB 206, designed by Novan, is an anti-viral topical gel that is developed to mitigate viral skin infections, including external genital and perianal warts resulting from HPV. This medication works by releasing nitric oxide (NO), which directly attacks and destroys the HPV responsible for genital warts, effectively inhibiting viral replication and potentially preventing recurrence by exerting a viricidal effect at the site of infection. Essentially, the NO released by SB 206 specifically targets and kills the virus that causes the warts.
VP-102: Verrica Pharamceuticals
VP-102, developed by Verrica Pharmaceuticals, is a topical drug-device combination containing cantharidin for the treatment of genital warts. Its mechanism of action involves inducing controlled blister formation by disrupting desmosomal cell adhesion in HPV-infected skin. Cantharidin, a vesicant, penetrates the epidermis and causes keratinocyte apoptosis, leading to the wart’s natural detachment. This targeted destruction of infected tissue helps eliminate HPV-infected cells while minimizing systemic absorption. VP-102’s precise application through its proprietary applicator ensures controlled dosing, reducing the risk of surrounding tissue damage.
| Drug Name | Company Name | MOA | ROA |
| SB 206 | Novan | Androgen receptor antagonists; Angiogenesis inducing agents; Cell death stimulants; Guanylate cyclase stimulants; Interleukin 13 inhibitors; Interleukin 4 inhibitors; Nitric oxide donors; NLRP3 protein inhibitors; Reactive oxygen species modulators | Topical |
| VP-102 | Verrica Pharamceuticals | Serine protease stimulants | Topical |
Leading Companies in the Genital Warts Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global genital warts market, several leading companies are at the forefront of developing integrated platforms to enhance the management of genital warts. Some of the major players include 3M Pharmaceuticals, Merck and Co., and Aresus Pharma. These companies are driving innovation in the genital warts market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for the illness.
In September 2024, Merck publications' revealed positive top-line Phase 3 trial (V503-064) outcomes for GARDASIL 9 (Human Papillomavirus 9-valent Vaccine, Recombinant) when used as a 9-valent Human Papillomavirus vaccine in Japanese males between 16 and 26 years old. The trial successfully met both its primary and secondary objectives by showing GARDASIL 9 administered in three doses reduced the development of persistent nine-HPV strain infections in genitourinary sites versus placebo dosing.
Request for customization: https://www.imarcgroup.com/request?type=report&id=7168&flag=E
Key Players in Genital Warts Market:
The key players in the Genital Warts market who are in different phases of developing different therapies are Novan, 3M Pharmaceuticals, Aresus Pharma, Orgenesis, Epitome Pharmaceuticals, Merck and Co., G&E Herbal Biotechnology, Verrica Pharamceuticals, and Others.
Regional Analysis:
The major markets for genital warts include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for genital warts while also representing the biggest market for its treatment. This can be attributed to the rising prevalence of human papillomavirus infections, advancements in treatment options, increasing awareness and vaccination programs, and strong healthcare infrastructure.
Moreover, a major driver is the widespread adoption of HPV vaccination programs, particularly with Gardasil 9, which protects against multiple HPV strains, including those responsible for genital warts. Increased vaccine coverage has contributed to a decline in new cases, yet the need for effective treatments remains due to the millions already infected.
Besides this, pharmaceutical advancements, including topical therapies like imiquimod, sinecatechins (Veregen), and innovative treatments like VP-102 (cantharidin-based therapy), are expanding market opportunities. The rise of minimally invasive procedures such as cryotherapy and laser ablation has also improved patient outcomes, increasing demand for specialized treatments.
Recent Developments in Genital Warts Market:
- In January 2024, Verrica Pharmaceuticals Inc. announced the receival of minutes from the Company's latest Type C meeting with the United States FDA, which occurred on November 6, 2023, for reviewing the Phase 3 clinical development strategy for YCANTH for common warts treatment.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the genital warts market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the genital warts market
- Reimbursement scenario in the market
- In-market and pipeline drugs
This report offers a comprehensive analysis of current genital warts marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
IMARC Group Offer Other Reports:
Primary Immune Deficiency Market: The 7 major primary immune deficiency markets reached a value of US$ 5.7 Billion in 2023, and projected the 7MM to reach US$ 9.4 Billion by 2034, exhibiting a growth rate (CAGR) of 4.61% during 2024-2034.
Cytomegalovirus Infections Market: The 7 major cytomegalovirus infections markets are expected to exhibit a CAGR of 4.32% during 2024-2034.
Viral Hepatitis Market: The 7 major viral hepatitis markets are expected to exhibit a CAGR of 2.69% during 2024-2034.
Hepatitis C Market: The 7 major hepatitis c markets reached a value of US$ 18.0 Billion in 2023, and projected the 7MM to reach US$ 47.1 Billion by 2034, exhibiting a growth rate (CAGR) of 9.14% during 2024-2034.
Hepatitis D Market: The 7 major hepatitis D markets reached a value of US$ 665.1 Million in 2023, and projected the 7MM to reach US$ 845.3 Million by 2034, exhibiting a growth rate (CAGR) of 2.2% during 2024-2034.
Hepatic Encephalopathy Market: The 7 major hepatic encephalopathy markets reached a value of US$ 1.7 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 2.9 Billion by 2034, exhibiting a growth rate (CAGR) of 5.1% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800