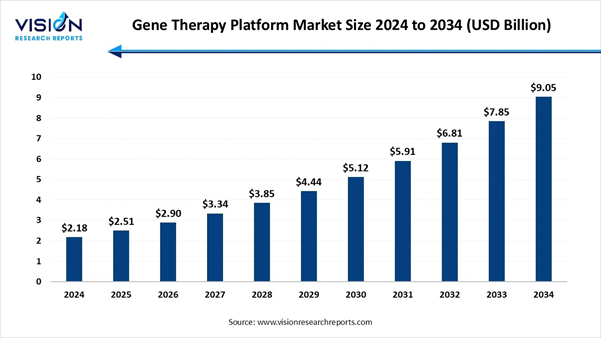
The global gene therapy platform market size was calculated at USD 2.18 billion in 2024 and is anticipated to hit USD 2.51 billion in 2025 to reach nearly USD 9.05 billion by 2034, expanding at an impressive CAGR of 15.3% from 2025 to 2034, a study published by Vision Research Reports.
The market demand is due to overall or off-the-shelf gene therapy products that could usually lower costs and develop accessibility, which opens up the latest market segments.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Preview the Report Before You Buy – Get Sample Pages 👉 https://www.visionresearchreports.com/report/sample/41678
What is the Gene Therapy Platform Market?
The gene therapy platform market offers products, services, technologies, tools, and procedures that are utilized to develop, discover, manufacture, and deliver gene-dependent therapeutic diagnosis. The aim of a stage catch is to make a standardized, reproducible, and scalable machine that can be used for a wide range of diseases to develop new therapies. This has the capability to restore function in cells by serving genetic material to particular cells that have genetic variants. Instead of treating chronic symptoms with medications over a lifetime period, such therapies' goal is to manage the underlying genetic cause of the condition with one or fewer treatments over time.
Gene
Therapy Platform Market Key Highlights: •
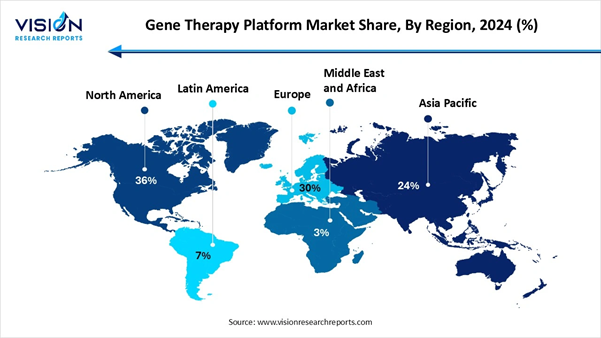
By region, North America accounted for the largest market share with 36% in
2024. •
By region, the Asia-Pacific region is projected to experience the fastest
growth rate over the forecast period. •
By product type, the viral vector platforms segment led the market with a 59%
share in 2024. •
By product type, the gene editing platforms segment is expected to see the
fastest growth during the forecast period. •
By application, oncology was the leading application in the market in 2024. •
By application, the hematological disorders segment is anticipated to grow at
the highest rate. •
By delivery method, the in vivo gene therapy segment captured the largest
revenue share in 2024. •
By delivery method, the ex vivo gene therapy segment is expected to experience
the most significant growth starting this year. •
By end use, pharmaceutical and biotechnology companies were the dominant
players in the market. •
By end use, contract research organizations are expected to be the
fastest-growing segment. Latest
Trends in the Gene Therapy Platform Market: •
Regional Collaboration for faster approvals: Action like The Collaboration on Gene
Therapies Global Pilot allows partnership checking of the gene therapy uses in
Canada, the U.S, and other countries, capable of developing the rollout of
diagnosis. Also, the European Medicines Agency (EMA) is giving importance to
high-level therapy medicinal products that develop conditional uses for
promising therapies. •
Lifetime value assessments:
Standard efficient patterns frequently fail in order to link to the lifelong
advantages of curative treatments. Rising trends include evaluating therapies
depending on the “lifetime value”. For instance, health economists are using
metrics like Quality Adjusted Life Years to perfectly quantify the value of
durable therapies. •
Value-based agreements: Value-dependent
payment patterns are gaining attention in the gene and cell therapy area. These
partnerships connect the reimbursement spaces to the therapy’s actual-world
performance. •
Advanced gene therapy manufacturing models: Automation and AI are updating
production, saving time and cutting costs while opening the latest
opportunities for scalability. Decentralized production models, such as local
production hubs, are leading to a lessening of logistical hurdles and
increasing accessibility globally. •
Patient advocacy and real-world collaboration: The position of patient advocacy in
terms of market access cannot be completely understood. Patient registries and
advocacy groups are serving invaluable data points about rare diseases that
assist in expanding the case for demonstrating long-term value to payers. Discover
the Full Market Insights 👉 https://www.visionresearchreports.com/gene-therapy-platform-market/41678 Gene
Therapy Platform Market Major Insights in 2025 Category Details Major
Applications Gene
therapy carries a perfect capability for diagnosing different types of
genetic conditions like as challenges of safety, delivery and efficiency
which are overcome. Conditions like sickle cell anemia and beta-thalassemia
have already witnessed promising outcomes with gene editing approaches. Market
Potential It
is projected at approximately USD 18 billion to around USD48 Billion by 2030
that relies on the methodology and scope. The gene therapy platform market
has main potential which is driven by the technological advancements and the
developing importance of cancer and genetic disorders. Key
Strategic drivers A
gene therapy platform is generally being driven by the potential to solve the
root genetic reason of disease and serve an scalable, reproducible and
acceptable manufacturing procedure. Critical
Challenges The
huge spread usage of cell and gene therapy generating gets limited by three
main elements that count regulatory complexities and high production costs
with restricted scalability. Current automation systems as well as AI and
bioprocessing technology develop potential and lower down expense too. Regional
Momentum The
main worldwide region displays rigid, but different, momentum for the gene
therapy stage ,which is being driven by technological advancements,
investments and developing regulatory frameworks. North America stayed the
market leader in terms of revenue, while the Asia-Pacific region is
witnessing the fastest growth.
Gene
Therapy Platform Market Opportunity What
Holds the Largest Potential in Gene Therapy Platform Market? The
gene therapy platform showcases strong expectations of exponential expansion as
pharmaceutical organizations, along with government and biotech firms, plan to
keep investing. Research shows that cell and gene therapy will develop into
tailored medical practices because new, effective, focused diagnosis options
will penetrate the market. The growing urge put demanding needs on the sector
in order to stay constant. The
global nations are growing their resources regarding high-level therapy
production facilities with the aim of developing efficiency and lowering
manufacturing expenses. Personalized production rules, collectively with the AI
optimization and automated manufacturing machine, will allow manufacturers to
produce sufficient products for developing patient numbers. Gene
Therapy Platform Market Key Challenges Production
Complexities to Create Hurdles for the Industry The
challenges in gene therapy display vector-related issues, production
complexities, logical hurdles, and safety issues like off-target effects in
terms of clinical development. These problems affect the effectiveness,
delivery, and scalability of treatments. The selection between viral and
non-viral vectors counts tradeoffs. Viral vectors like adeno-associated virus
(AAV) and lentivirus are highly efficient but have perfect size restrictions
and safety risks, too. Gene
Therapy Platform Market Report Coverage Report Attribute Key Statistics Market Size in 2025 USD 2.51 Billion Market Size in 2026 USD 2.90 Billion Market Size in 2030 USD 5.12 Billion Market Size in 2032 USD 6.81 Billion Market Size by 2034 USD 9.05 Billion Growth rate from 2025 to
2034 CAGR of 15.3% Base Year 2024 Forecast Period 2025 to 2034 Segments Covered By Platform Type, By
Therapeutic Application, By Delivery Mode, By End Use Companies Covered Novartis AG, Gilead
Sciences (Kite Pharma), Spark Therapeutics (Roche), Bluebird Bio, REGENXBIO,
Sarepta Therapeutics, UniQure N.V., Audentes Therapeutics (Astellas Pharma),
and Amicus Therapeutics.
For
Orders or Inquiries, Don’t Hesitate to Reach Out: sales@visionresearchreports.com Gene
Therapy Platform Market Regional Analysis How
did the North America Region Dominate the Gene Therapy Platform Market? The
North America region has dominated the market in 2024 as several stages of
processes are being made available in order to discover manufacturing and
growth of the viral vectors. There is a main focus on manufacturing and
chemistry controls (CMC), and there is rigid importance in collaborating with
experienced CDMOs and CRO’s for the testing. There is also higher data from the
clinical trials that track adverse
reactions. The application of AI for the retrospective data analysis of all
clinical and preclinical data will assist in the perfect design of the clinical
growth process and clinical trials. With
Instance, •
In March 2025, LUXTURNA has the main market capability because of high unmet
demand for smooth treatments in rare genetic conditions. It is officially being
approved by the FDA for particular mutations in the RPE65 gene, as it serves
hope to patients who previously had limited options. (Source: https://www.prnewswire.com) •
In the year 2024, the U.S Food and Drug Administration officially reported 3
approved CGT products. The different conditions that are being treated by cell
therapies include several types of cancer, including lymphoblastic leukemia,
large B-cell lymphoma, and several myeloma. Mexico:
Key Growth Drivers in the Gene Therapy Platform Market: •
Development of biotech capabilities: Mexico is improving its biotech
infrastructure, which includes expertise and facilities, in order to assist
advanced therapeutic development. •
Biotech investments: The
country is experiencing funding in its biotech sector from both private and
public entities. •
Growing healthcare spending: Growing healthcare spending is developing permission to
high-level medical treatments and boosting the overall market. •
Rising disease commonness: Mexico,
just like other countries, is witnessing a rising incidence of chronic
illnesses such as cancer and genetic disorders. This grows the urge for
targeted treatments and novel gene therapies. •
Inventions in manufacturing: Mexico is starting to benefit from global innovations in
terms of gene therapy production procedures that include continuous automation
and manufacturing with developed efficiency. Why
is Asia Pacific the Fastest-Growing region in the Gene Therapy Platform Market? The
Asia-Pacific region is expected to reach huge development as it shows elements
that count the developing load of the chronic disease, the growing number of
positive clinical trials, and the development in gene therapy-based biotech
companies. The APAC region is a centre for clinical trials with a large and
diverse patient population. The number of cell and gene therapy clinical trials
in the region has been developing mainly faster than in the rest of the world.
Also, China alone accounts for a big percentage of global cell therapy trials. Pharmaceutical
companies and governments across the regions are significantly contributing to
gene therapy research and development. Public-private collaboration and biotech
startup investments are also developing manufacturing and infrastructure
capacity. India:
Key Growth Drivers in the Gene Therapy Platform Market: •
Strategic initiatives: The
Indian government is actively marketing indigenous research and biotechnology
through initiatives like "Make in India“ and investment from agencies such
as the Department of Biotechnology and the Indian Council of Medical Research
(ICMR). •
First-homegrown therapy: In
a major milestone, India revealed its primary indigenous CAR-T cell therapy for
cancer in April 2024. This achievement highlights the capability for
academia-sector collaboration in order to generate affordable therapies. •
Dedicated Funding: The
DBT has assisted over 80 R&D projects that concentrate on genome editing
technologies for healthcare uses. A multi-million dollar national aim for the
CGT and focused research grants are being further developed. •
Evolving regulatory framework: The central Drug Standard Control Organization has made
a clearer path for the gene therapy items under the New Drugs and Clinical
Trial Rules in 2019. The ICMR has also generated national guidelines that serve
as a precise regulatory and ethical framework. For
Instance, ● In November 2025, Bharat
Biotech International Ltd disclosed the formal launch of Nucelion Therapeutics
Pvt Ltd, which marks a penetration into cell and gene therapies that focus on
diagnosing autoimmune disorders, cancers, and rare genetic diseases. (Source: https://newsmeter.in) Need
a Tailored Version of the Report? | Get Customization Options Here: https://www.visionresearchreports.com/report/customization/41678 Gene
Therapy Platform Market Segmentation Analysis Platform
Type Analysis Why
did the viral vector platforms segment dominate the gene therapy platform
market? The
viral vectors have dominated the gene therapy platform in 2024, as it has become famous in terms of
gene therapy because of their natural potential to deliver genetic material
smoothly into human cells, which solves the fundamental issues of getting
therapeutic genes to the correct place. The
regulatory successes of viral vectors can be focused on many advantages over
non-viral delivery methods. They have the potential of both long-term transgene
expression for the chronic conditions and short-term level surface that is
perfect for cancer therapies. They
can even fight abilities in order to regulate or end gene expression, as well
as the degree of control for tissue-specific targeting. This potential for adaptable
and accurate gene delivery underlines their developing use in both in vivo and
ex vivo therapeutic methods. The
gene editing segment is predicted to rise at the fastest rate. Gene-editing technologies
have usually stretched the therapeutic capability of gene therapy, which allows
diagnosis for a much wider range of diseases. Just like regular gene therapies,
which can reveal a regular copy of a missing or defective gene, the
gene-editing tools enable the direct removal, correction, and modification of
the aberrant genes within the patient’s own DNA. Application
Analysis Why
did the oncology segment dominate the gene therapy platform market? The
oncology segment has dominated the gene therapy segment in 2024 as it became a famous stage for
gene therapy because of the main factors that include the urgent, unmet medical
demand for new cancer diagnosis and cancer; different characteristics which
make it a perfect and feasible target. Cancer is classified by fast, uncontrolled
cell proliferation. These characteristics made it an initial target for the
therapies that focus on particular attacks for fast-dividing cells, which was
an ideal first discovery with crafted viruses and suicide genes. The
hematological disorders segment is expected to rise at the fastest rate. Hemoglobinopathies, such
as sickle cell disease (SCD) and beta thalassemia, are the main targets for
gene therapy because of the dramatic improvements witnessed with even minute
increases in normal globin chain manufacturing or the reactivation of the fetal
hemoglobin. Current FDA approvals feature development like Casgevy, which
employs CRISPR/Cas 9, which updates hematopoietic stem cells in order to manage
SCd with recurrent pain crises and transfusion-dependent beta-thalassemia. Delivery
Method Analysis Why
did the in vivo gene therapy segment dominate the gene therapy platform market? The
in vivo gene therapy has dominated the market in 2024 as it has gained attention
due to the development in viral vectors, as it has displayed success in
diagnosing certain diseases, and the logistical and production challenges
inherent to its main part. In vivo delivery is the selected or only option for
the diseases that affect organs that are difficult or impossible to remove,
return, or modify, such as the brain, eye, and heart. In vivo therapy by the
opposite is mostly limited to permissible tissues like immune cells and blood,
too. The
ex vivo gene therapy segment is expected to rise at the fastest rate. The ex vivo gene therapy
is important by serving as a safer, more manageable method in order to treat
particular diseases, which solves a main challenge faced by early in vivo
strategies. By updating a patient's cells outside the body, researchers can
make sure that the genetic changes are correct before turning the cells back to
the patient, thereby lowering the risk of bad immune feedback and unifying
genetic updates. This success laid the basis for the huge gene therapy stage by
serving the idea and updating the complicated technologies needed for genetic
engineering. End-use
Analysis How
did the pharmaceutical and biotechnology segment dominate the gene therapy
platform market? The
pharmaceutical and biotechnology segment had dominated the market in 2024 as pharmaceutical and biotechnology organizations have become
dominant in terms of gene therapy due to innovative technological advances, and
moved towards tailored medicine that focuses on the genetic root of the
diseases. After years of feedback and slow clinical development, the field is
mature enough for a strong commercial platform that is driven by both nimble
biotech startups and big pharmaceutical firms. Also,
the biotech forms that concentrate on making and updating vector technologies
and gene-editing stages, like CRISPR. For instance, organizations like uniQure
and Spark Therapeutics were early pioneers in particular gene therapies. The
contract research organizations segment is expected to rise at the fastest
rate. The
contract research organization is highly diversified with both niche
specialists and full-service providers in particular therapeutic areas,
geographies, and phases of the development. As invention moves towards biologics, gene therapies, and precision medicine, CROs are developing to
serve advanced capabilities in spaces like real-world proof, digital health, and decentralised trials.
The
growth of specialised therapies, such as cell and gene therapies, and tailored
medicine is encouraging sponsors to find CRO expertise. The development of CRO
is being grown into areas like real-world evidence, decentralized trials
collection, and data analytics. Browse
More Related Insights: •
U.S. Cell And Gene Therapy Manufacturing Market: https://www.visionresearchreports.com/us-cell-and-gene-therapy-manufacturing-market/41265 •
Cancer Gene Therapy Market: https://www.visionresearchreports.com/cancer-gene-therapy-market/39503 •
Particle Therapy Market: https://www.visionresearchreports.com/particle-therapy-market/41373 •
Stem Cell Therapy Market: https://www.visionresearchreports.com/stem-cell-therapy-market/41342 •
U.S. Cell Therapy Market: https://www.visionresearchreports.com/us-cell-therapy-market/41239 • Cell Therapy Raw Materials Market: https://www.visionresearchreports.com/cell-therapy-raw-materials-market/40883 Recent
Developments in the Gene Therapy Platform Market •
In August 2025, Klotho Neurosciences, Inc. revealed that it had signed a
binding agreement to start development and production of its KLTO-202 gene
therapy candidate, which uses the AAVnerGene Inc. (Source: https://www.prnewswire.com) •
In October 2025, Krystal Biotech received a stage technology designation from
the FDA for the use of genetically updated, nonreplicating herpes simplex virus
type 1 (HSV-1), which is a viral vector that is being utilized in KB801, an
investigational gene therapy designed to diagnose neurotrophic keratitis. (Source:
https://www.cgtlive.com) •
In August 2025, Andelyn Biosciences, Inc. is a top and patient-centric cell and
gene therapy Contract Development and Manufacturing Organization (CDMO) that
has collaborated with AMplo Biotechnology, an innovative company in terms of
adeno-associated virus (AAV), which is regenerative medicine for the
neuromuscular junction, affecting conditions. (Source: https://www.prnewswire.com) Top
Companies in the Gene Therapy Platform Market: •
Gilead Sciences, Inc •
Novartis AG •
Spark Therapeutics, Inc •
Regenxbio Inc •
Bluebird Bio, Inc. •
Sarepta Therapeutics, Inc •
Audentes Therapeutics, Inc •
UniQure N.V •
REGENXBIO Inc •
Amicus Therapeutics, Inc Gene Therapy Platform Market
Segmentation By
Platform Type •
Viral Vector Platforms • Adeno-associated Virus (AAV) • Lentivirus • Retrovirus • Adenovirus • Herpes Simplex Virus (HSV) •
Non-Viral Vector Platforms • Lipid Nanoparticles (LNPs) • Electroporation & Microinjection Platforms • Polymer-based Delivery Systems • Naked DNA/RNA Delivery •
Gene Editing Platforms • CRISPR-Cas Systems • TALENs • ZFNs By
Application •
Oncology •
Rare Genetic Disorders •
Cardiovascular Diseases •
Neurological Disorders •
Ophthalmic Diseases •
Hematological Disorders (e.g., Hemophilia, Sickle Cell) •
Musculoskeletal Disorders •
Infectious Diseases (e.g., HIV, COVID-19 adjunct therapies) By
Delivery Mode •
In Vivo Gene Therapy •
Ex Vivo Gene Therapy • Autologous Cell-Based Gene Therapy • Allogeneic Cell-Based Gene Therapy •
Others (In-situ Gene therapy) By
End Use •
Pharmaceutical & Biotechnology Companies •
Academic & Research Institutions •
Contract Development & Manufacturing Organizations (CDMOs) •
Hospitals & Gene Therapy Centers By
Region •
North America •
Europe •
Asia Pacific •
Latin America •
Middle East and Africa Instant
Delivery Available | Purchase This Exclusive Research Report Now: https://www.visionresearchreports.com/report/checkout/41678 You can place an order or ask any
questions, please feel free to contact at: sales@visionresearchreports.com About
Us Vision
Research Reports is a premier service provider offering strategic market
insights and solutions that go beyond traditional surveys. We specialize in
actionable market research, delivering in-depth qualitative insights and
strategies to global industry leaders and executives, helping them navigate
future uncertainties. Our offerings include consulting services, syndicated
market studies, and bespoke research reports. We
are committed to excellence in qualitative market research, fostering a team of
experts with deep industry knowledge. Our goal is to help clients understand
both current and future market trends, empowering them to expand their
portfolios and achieve their business objectives with the right guidance. Web: https://www.visionresearchreports.com Our
Trusted Data Partners Precedence Research | Statifacts | Nova One Advisor For
Latest Update Follow Us: LinkedIn