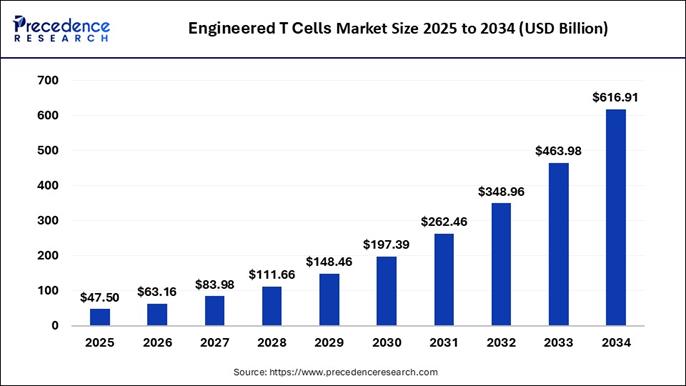
According to Precedence Research, the global engineered T cells market size is expected to be worth over USD 616.91 billion by 2034, increasing from USD 47.5 billion in 2025, growing at a strong CAGR of 32.96% between 2025 and 2034.
The global engineered T cells market size is valued at USD 47.5 billion in 2025 and it is projected to grow from USD 63.16 billion in 2026 to approximately USD 616.91 billion by 2034. In terms of CAGR, the market is expanding at a compound annual growth rate (CAGR) of 32.96% between 2025 and 2034. This rapid growth is driven by advancements in cell engineering technologies, rising prevalence of cancer and autoimmune disorders, and increasing clinical adoption of CAR-T and TCR-T therapies.
North America currently dominates the market, supported by strong biotechnology infrastructure and high research investment, while Asia-Pacific is emerging as a key growth hub with expanding clinical capabilities and government support. Leading players such as Novartis, Gilead, Bristol-Myers Squibb, and Amgen are driving innovation in engineered T cell therapy platforms aimed at enhancing safety, scalability, and therapeutic efficacy.
This Report is Readily Available for Immediate Delivery | 📥 Download the Sample Pages of this
Report@ https://www.precedenceresearch.com/sample/3224
Engineered T Cells Market Highlights:
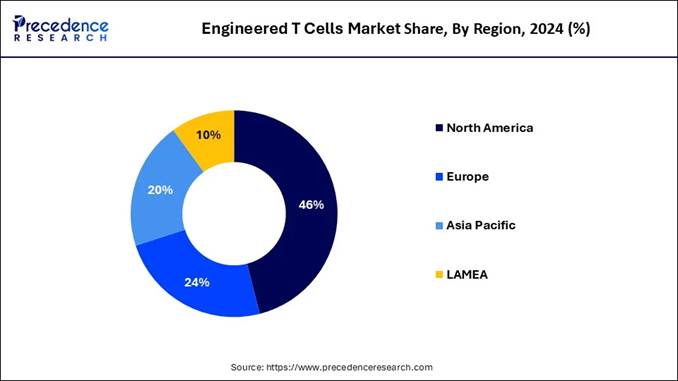
➢ North America dominated the market, capturing approximately 46% of market share in 2024.
➢ By type, the chimeric antigen receptor (CAR) modified T cells segment accounted for the highest market share of 78% in 2024.
➢ By end-user, the hospitals segment held the major market share of 58% in 2024.
➢ By application, the lung cancer segment is expected to expand at a notable CAGR from 2025 to 2034.
Engineered T Cells Market Report Coverage
|
Category |
Details |
|
Market Size in 2024 |
USD 35.73 Billion |
|
Market Size in 2025 |
USD 47.5 Billion |
|
Market Size in 2026 |
USD 63.16 Billion |
|
Market Size in 2030 |
USD 197.39 Billion |
|
Market Size in 2034 |
USD 616.91 Billion |
|
Growth Rate (2024–2034) |
CAGR of 32.96% |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2034 |
|
Leading Region (2024) |
North America (46% market share) |
|
Dominant Segment by Type (2024) |
Chimeric Antigen Receptor (CAR) Modified T Cells – 78% share |
|
Dominant Segment by End-user (2024) |
Hospitals – 58% share |
|
Leading Application Segment (2024) |
Lung Cancer |
|
Key Growth Drivers |
Rising cancer prevalence, increasing clinical adoption of CAR-T and TCR-T therapies, advancements in gene-editing (CRISPR), growing biotech investments |
|
Key Challenges |
High therapy and research costs, complex manufacturing, need for skilled professionals and sterile facilities |
|
Regional Highlights |
North America leads with advanced infrastructure and high R&D funding; Asia-Pacific shows fastest growth due to expanding cell therapy programs in China, Japan, and India |
|
Top Companies |
Novartis AG, Gilead Sciences Inc., Bristol-Myers Squibb, Amgen Inc., Eli Lilly and Company, Pfizer Inc., Bellicum Pharmaceuticals, Precision BioSciences, Oxford Biomedica, Athenex Inc. |
|
Regulatory Authorities |
FDA (U.S.), EMA (EU), NMPA (China) |
|
Major Regulations |
FDA: PHS Act Section 351; EMA: ATMP Regulation (EC) No 1394/2007; NMPA: Drug Administration Law |
|
Regions Covered |
North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
|
Segmentation Covered |
Type, Application, End-user, Region |
Engineered T Cells Market Overview and Industry Potential
Reprogramming Immunity: Engineered T Cells Redefine the Future of Cancer Therapy
The engineered T cells market is anticipated to experience significant growth in the coming years, akin to technology advances where the doctor can design patients' own immune cells to fight cancer and other diseases. Moreover, by finding and destroying cancer cells, the engineered T cells have gained major attention from global investors and corporate backers in recent years.
✚ Browse Detailed Insight 👉https://www.precedenceresearch.com/engineered-t-cells-market
Market Potential: From Discovery to Demand: Expanding Therapeutic Reach in Critical Illness
The shift towards lung, breast, and brain cancers is expected to create lucrative opportunities for the industry in the coming years. Moreover, several researchers are seen under the heavy testing and development, which can gain a major industry share during the forecast period. Also, the expansion in autoimmune and viral diseases, the developers expect to gain a heavy consumer base in the coming years as per the future industry expectations.
Market’s Challenge: Sterile Labs and Skilled Labor Add to the Financial Burden
The high cost of research and complex process is anticipated to hinder the industry's growth during the projected period. Also, the procedure needs sterile labs, strict storage conditions, and a skilled expert for the individual patient, which can create cost challenges for patients and companies.
Engineered T Cells Market’s Regulatory Landscape: Global Regulations
|
Country / Region |
Regulatory Body |
Key Regulations |
Focus Areas |
Notable Notes |
|
United States |
Food and Drug Administration (FDA). |
Public Health Service Act (PHS Act) Section 351 |
Balancing efficacy and safety through rigorous clinical trials |
The agency is specifically the Center for Biologics Evaluation and Research (CBER), which is responsible for the oversight of cellular and gene therapy products. |
|
European Union |
European Medicines Agency (EMA) |
Regulation (EC) No 1394/2007 on Advanced Therapy Medicinal Products (ATMPs) |
Establishing a clear framework for ATMPs |
This agency conducts a centralized scientific evaluation of marketing authorization applications for advanced therapies, valid across all EU member states. |
|
China |
National Medical Products Administration (NMPA). |
"Drug Administration Law (DAL) |
Accelerating clinical development and approval of innovative therapies to meet patient needs |
Responsible for the registration, approval, and regulation of drugs and medical devices, including engineered T-cell therapies. |
➡️ Become a valued
research partner with us ☎
https://www.precedenceresearch.com/schedule-meeting
Case Study: CAR-T Therapy Transformation — Novartis’ Kymriah Leading the Engineered T Cells Revolution

Background:
Before the emergence of engineered T cells, late-stage blood cancers such as
acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL)
had limited treatment options, often leading to poor survival outcomes. The
introduction of chimeric antigen receptor (CAR)-modified T cells marked
a paradigm shift in cancer immunotherapy by enabling
physicians to reprogram a patient’s own immune cells to recognize and destroy malignant
cells.
Case Overview – Novartis Kymriah:
Novartis AG became the first company to commercialize a CAR-T therapy with Kymriah
(tisagenlecleucel), approved by the U.S. Food and Drug Administration (FDA) in
2017. Kymriah is indicated for pediatric and young adult patients with relapsed
or refractory B-cell ALL, and later for adult patients with DLBCL.
The therapy involves extracting a patient’s T cells, genetically engineering
them to express a chimeric antigen receptor (CAR) that targets the CD19 protein
on cancer cells, expanding these modified cells in sterile manufacturing
facilities, and then infusing them back into the patient.
Implementation and Manufacturing
Innovation:
Novartis established a global network of specialized manufacturing sites in the
U.S., Europe, and Asia to support large-scale CAR-T production. The process
required ultra-sterile laboratories, cryogenic storage systems, and rigorous
regulatory oversight.
To overcome the bottleneck of individualized cell manufacturing, Novartis
introduced digital chain-of-identity systems ensuring traceability from cell
collection to reinfusion, maintaining patient safety and data integrity.
Clinical Outcomes:
Clinical trials demonstrated
remarkable remission rates: in the pivotal ELIANA study, 83% of pediatric and
young adult patients achieved complete remission within three months of
treatment. These results not only validated the scientific foundation of
engineered T cells but also paved the way for global acceptance of personalized
immunotherapies.
Market Impact:
Kymriah’s success triggered a surge in investment and innovation across the cell and gene therapy ecosystem. Competitors like Gilead’s Yescarta and Bristol-Myers
Squibb’s Breyanzi soon entered the market, expanding the scope of
engineered T cell therapies into various cancer types.
This commercial validation established the engineered T cells market as
a core growth pillar within oncology—contributing significantly to the market’s
projected expansion from USD 47.5 billion in 2025 to USD 616.91
billion by 2034, growing at a CAGR of 32.96%.
Strategic Lessons for the Industry:
- Regulatory alignment and early collaboration with agencies such as the FDA and EMA were critical in accelerating clinical approvals.
- Infrastructure scalability—including cryogenic logistics, contamination control, and automated production lines—proved essential for commercial viability.
- Cross-sector partnerships among biotech firms, academia, and contract manufacturers reduced development timelines and enhanced technical expertise.
- Patient-centric care models and data-driven follow-up programs built trust and improved long-term outcomes.
Conclusion:
The Kymriah case underscores how engineered T cell therapy has redefined cancer
treatment through personalized, living medicines. It illustrates the transformative impact of biotechnology innovation and provides a roadmap for emerging players aiming to expand the
reach of cellular immunotherapies.
As multiple CAR-T and TCR-T programs move toward commercialization across solid
and hematologic cancers, the global engineered T cells market stands as a
testament to the convergence of genetic engineering, precision medicine, and
large-scale biomanufacturing.
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/3224
Engineered T Cells Market Key Regional Analysis:
How North America Leads the Global Engineered T Cells Market?
North America held the dominant share of the engineered T cells market in 2024, owing to having access to advanced technology and advanced biotechnology research. Moreover, the region has seen under higher healthcare spending and early adoption of cell therapies. Also, the clinical trial centres are heavily contributing to the industry growth in the region in the current period.
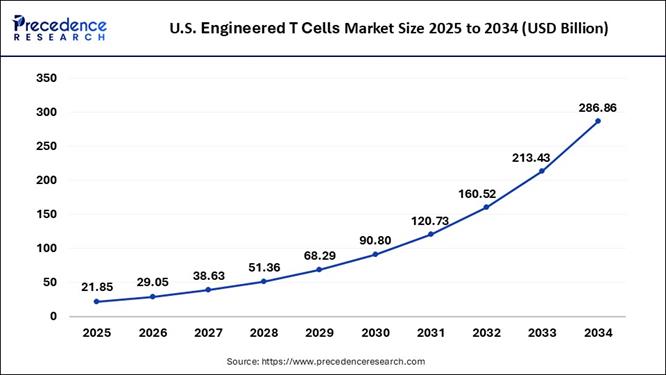
What is the U.S. Engineered T Cells Market Size and Growth Rate?
According to Precedence Research, the U.S. engineered T cells market size is estimated at USD 21.86 billion in 2025 and is projected to exceed USD 286.86 billion by 2034. The industry is accelerating a double-digit compound annual growth rate (CAGR) of 29.37% from 2025 to 2034.
United States Engineered T Cells Market Analysis:
The United States engineered T cells market is witnessing robust growth, driven by increasing advancements in immunotherapy and the growing prevalence of cancer and infectious diseases. Engineered T cell therapies—such as CAR-T (Chimeric Antigen Receptor T-cell) and TCR-T (T Cell Receptor-engineered T-cell) therapies, have revolutionized cancer treatment by offering personalized and highly targeted approaches that harness the body’s own immune system.
Major factors fueling the market include rising investments in biotechnology research, expanding FDA approvals for new cell-based therapies, and increasing collaborations between biotech firms and academic institutions. The market also benefits from a favorable regulatory landscape and accelerated clinical trials.
Note: This report is
readily available for immediate delivery. We can review it with you in a
meeting to ensure data reliability and quality for decision-making.
📥 Download
Sample Pages for Informed Decision-Making 👉 https://www.precedenceresearch.com/sample/3224
Is Asia the Next Global Hub for Innovation?
Asia Pacific is expected to expand notably during the forecast period, because countries like China, Japan, and South Korea are rapidly expanding cell therapy research and manufacturing capacity. Asian governments are offering funding, fast regulatory approvals, and local production incentives.
Furthermore, the presence of a large patient pool and lower therapy development costs make the region an attractive hub for clinical research. As awareness of personalized medicine grows, Asia-Pacific is poised to emerge as one of the fastest-growing regions in the global engineered T cells market over the next decade.
India Engineered T Cells Market:
🔹In India, the engineered T cells market is in an emerging phase but gaining momentum rapidly due to increasing cancer incidence, growing biomedical research capacity, and supportive government policies for biotechnology development.
🔹Indian research institutes and biotech startups are increasingly collaborating with international firms to establish CAR-T therapy manufacturing and clinical programs domestically.
🔹The government’s “Make in India” and “Atmanirbhar Bharat” initiatives are further fostering local production capabilities for advanced therapies. However, challenges such as high treatment costs and limited infrastructure for large-scale cell manufacturing persist.
🔹With ongoing clinical trials and regulatory progress, India is expected to evolve into a significant regional player in engineered T cell therapy in the coming years.
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Engineered T Cells Market Segmentation Analysis:
Type Analysis:
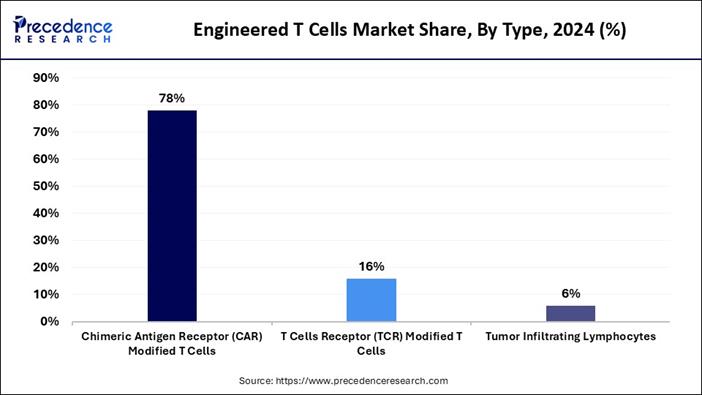
Why Did the Chimeric Antigen Receptor-Modified T Cells Segment Dominate the Market in 2024?
The chimeric antigen receptor-modified T cells segment held the largest share of the engineered T cells market in 2024, owing to its effectiveness in the treatment of blood cancers such as lymphoma and leukaemia. Moreover, the by identifying exact cancer targets while destroying tumours, the segment has gained major industry attention in recent years.
End User Type Analysis:
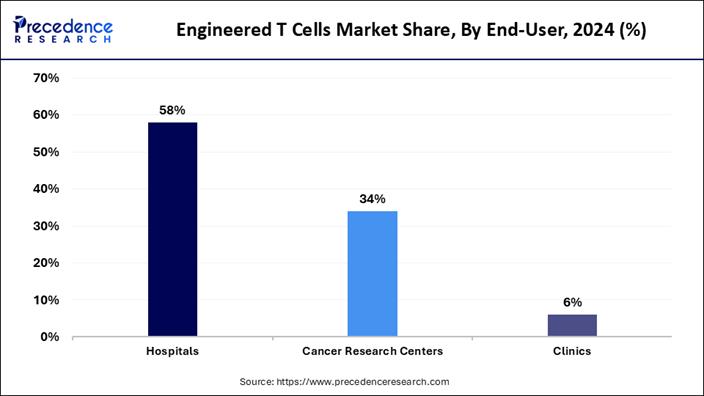
How the Hospital Segment Maintains Its Dominance in the Current Industry?
The hospital segment maintained the largest share of the market in 2024 because these treatments must be performed in specialized clinical settings with expert supervision. The process involves cell collection, modification, infusion, and post-treatment monitoring, all of which require advanced facilities and trained oncologists.
Application Analysis:
The lung cancer segment has gained a major share of the engineered T cells market in 2024, because it is one of the most common and deadliest cancers worldwide. Traditional treatments often fail at later stages, creating a big need for new options. Engineered T cells offer targeted therapy that can attack tumor cells without damaging healthy tissues.
Engineered T cell therapies are designed to precisely recognize and attack tumor-specific antigens present on lung cancer cells, enabling a highly selective and potent anti-tumor response while minimizing damage to surrounding healthy tissues. Advances in tumor antigen identification, gene-editing technologies, and delivery mechanisms have further enhanced the safety and efficacy profiles of these therapies.
✚ Related Topics You May Find Useful:
➡️ T-Cell Immunotherapy Market: Explore how next-generation immunotherapies are revolutionizing cancer treatment and reshaping precision medicine.
➡️ T-Cell Therapy Market: Analyze how engineered immune cells are driving breakthroughs in oncology and autoimmune disease management.
➡️ Regenerative Medicine Market: Understand how stem cell technologies and tissue engineering are redefining modern healthcare and recovery solutions.
➡️ Induced Pluripotent Stem Cells Market: Discover how iPSC innovations are advancing drug discovery, regenerative research, and disease modeling.
➡️ Primary Cell Culture Market: Learn how advancements in primary cell models are enhancing biomedical research accuracy and therapeutic testing.
➡️ Cell Therapy Manufacturing Market: Examine how automation, scalability, and regulatory frameworks are transforming global cell therapy production.
➡️ Cell and Gene Therapy Bioanalytical Testing Services Market: See how bioanalytical innovations are ensuring safety, efficacy, and compliance in advanced therapeutic development.
➡️ Cell Separation Market: Track how precision cell sorting technologies are enhancing clinical research, diagnostics, and personalized medicine.
Top Companies in Engineered T Cells Market & Their Offerings:
🔹 Amgen Inc. – Develops next-generation immunotherapies and engineered T cell platforms targeting cancer and autoimmune diseases.
🔹 Bellicum Pharmaceuticals Inc. – Focuses on controllable CAR-T and TCR-T cell therapies using proprietary switch technology for improved safety.
🔹 Bristol-Myers Squibb – Offers CAR-T therapies such as Breyanzi and Abecma, targeting hematologic malignancies.
🔹 Precision BioSciences Inc. – Utilizes ARCUS genome editing technology to develop off-the-shelf allogeneic CAR-T cell therapies.
🔹 Eli Lilly and Company – Expanding into cell therapy through acquisitions and collaborations to advance engineered T cell treatments.
🔹 Gilead Sciences, Inc. – Through its Kite Pharma subsidiary, leads in commercial CAR-T therapies for blood cancers.
🔹 Novartis AG – A pioneer in CAR-T therapy with Kymriah, focusing on personalized T cell treatments for cancer.
🔹 Athenex Inc. – Develops T cell receptor-engineered therapies for solid tumors and hematologic cancers.
🔹 Oxford Biomedica Plc – Provides viral vector manufacturing and cell therapy development services for engineered T cell products.
🔹 Pfizer Inc. – Invests in CAR-T and gene therapy collaborations to expand its oncology and cell-based immunotherapy portfolio.
What is Going Around the Globe?
🔹 In May 2023, Anixa Biosciences, Inc., a biotechnology company specializing in the diagnosis, treatment, and prevention of cancer, recently announced that it had started treating another patient in the current clinical study of its cutting-edge chimeric antigen receptor T-cell (CAR-T) therapy for ovarian cancer in collaboration with the institution it partners with Moffitt Cancer Center.
🔹In March 2023, the definitive agreement between Adaptimmune Therapeutics plc
and TCR2 Therapeutics Inc. was made public. It states that Adaptimmune would
merge with TCR2 in an all-stock transaction to become a world-class cell
therapy business specializing in solid tumors. The combination offers
significant product delivery and clinical development advantages, supported by
complementing technological frameworks. Therefore, the combined company's cash
runway is expected to increase after the merger closes through 2026.
Engineered T Cells Market Segmentation:
By Type
🔹Chimeric Antigen Receptor (CAR) Modified T Cells
🔹T Cells Receptor (TCR) Modified T Cells
🔹Tumor Infiltrating Lymphocytes
By Application
🔹Lung Cancer
🔹Colorectal Cancer
🔹Melanoma
🔹Breast Cancer
🔹Leukemia
By End-user
🔹Hospitals
🔹Cancer Research Centers
🔹Clinics
By Region
🔹North America
🔹Europe
🔹Asia-Pacific
🔹Latin America
🔹Middle East and Africa
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or Asia Pacific.
Don’t Miss Out! | Instant Access to This Exclusive Report 👉 https://www.precedenceresearch.com/checkout/3224
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344
Stay Ahead with Precedence Research Subscriptions
Unlock exclusive access to powerful market intelligence, real-time data, and forward-looking insights, tailored to your business. From trend tracking to competitive analysis, our subscription plans keep you informed, agile, and ahead of the curve.
Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription
About Us
Precedence Research is a global market intelligence and consulting powerhouse, dedicated to unlocking deep strategic insights that drive innovation and transformation. With a laser focus on the dynamic world of life sciences, we specialize in decoding the complexities of cell and gene therapy, drug development, and oncology markets, helping our clients stay ahead in some of the most cutting-edge and high-stakes domains in healthcare. Our expertise spans across the biotech and pharmaceutical ecosystem, serving innovators, investors, and institutions that are redefining what’s possible in regenerative medicine, cancer care, precision therapeutics, and beyond.
Web: https://www.precedenceresearch.com
Our Trusted Data Partners:
Towards Healthcare | Nova One Advisor | Onco Quant | Statifacts
Get Recent News 👉 https://www.precedenceresearch.com/news
For Latest Update Follow Us:




