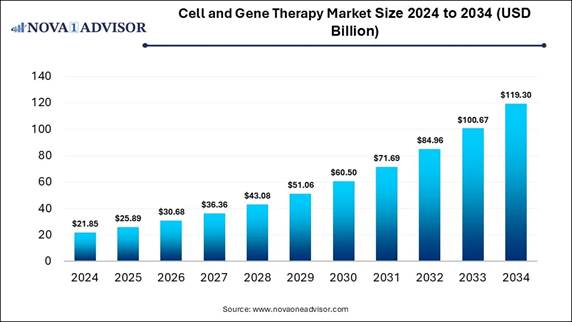
According to Nova One Advisor, the global cell and gene therapy market size is expected to be worth around 119.30 billion by 2034, increasing from USD 25.89 billion in 2025, representing a healthy CAGR of 18.5% from 2025 to 2034.
The cell and gene therapy market is growing, as these therapies are mainly focused on managing monogenic disorders produced by a mutation in a single gene, like SCID, muscular dystrophy, and haemophilia. CGT is increasing to target more complicated multigenic diseases like tumors, cardiac disease, and diabetes by changing the expression of multiple genes instantaneously.
The most frequent type of cell therapy is blood transfusion, and the transfusion of RBC, WBC, and platelets from a donor. Single therapies that restore healthy cellular function, eventually cultivating life expectancy and lowering the burden of chronic diseases.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/7720
Cell and Gene Therapy Market Highlights:
🔹By region, North America dominated the cell and gene therapy market with a revenue share of approximately 43% in 2024.
🔹By region, Asia Pacific is expected to grow at the fastest CAGR in the market during the forecast period.
🔹By therapy type, the cell therapy segment dominated the market with the highest revenue shares of approximately 55%.
🔹By therapy type, the gene therapy (in vivo) segment is expected to grow at the fastest CAGR in the Cell and Gene Therapy market during the forecast period.
🔹By vector type, the viral vectors segment held the highest revenue shares of approximately 68% in the market in 2024.
🔹By vector type, the non-viral vectors (LNPs) segment is expected to grow at the fastest CAGR in the market during the forecast period.
🔹By application, the oncology segment held the highest market share of approximately 47% in 2024.
🔹By application, the rare & genetic disorders segment is expected to grow at the fastest CAGR in the market during the forecast period.
🔹By end user, the pharmaceutical & biotechnology companies segment led the Cell and Gene Therapy market with the largest revenue share of approximately 41% in 2024.
🔹By end user, the CROs & CDMOs segment is expected to grow at the fastest CAGR in the market during the forecast period.
🔹By delivery mode, the ex vivo administration segment held the largest market share with the biggest revenue share of approximately 58% in 2024.
🔹By delivery mode, the in vivo administration segment is expected to grow at the fastest CAGR in the market during the forecast period.
🔹By industry vertical, the biotechnology segment held the largest market share with the biggest revenue share of approximately 37% in 2024.
🔹By industry vertical, the pharmaceuticals segment is expected to grow at the fastest CAGR in the market during the forecast period.
Market Overview and Industry Potential
Cell and gene therapies utilize cells and genes to treat genetic and rare diseases. This therapy has predominant potential to lay the foundation for the future big paradigm shift in drug development and manufacturing. It provides the ability for untreated syndromes or diseases caused by genetic alterations.
The development of cell and gene therapy is reforming drug discovery, offering new hope for rare diseases and cancer through advanced gene therapy. The potential of cell and gene therapies provides new management strategies for patients afflicted with genetic diseases.
With the rapid increase of cell and gene therapies over the last decade, biotech’s, biopharma, and nonprofits are taking on diseases that are conventionally complex to treat. Targeted cell and gene therapy has transformed precision medicine by enabling physicians to fix genetic defects that cause major illnesses.
Applications of Viral Vectors:
|
Application |
Function |
|
Sickle Cell Disease |
Correcting the underlying faulty genes |
|
Spinal Muscular Atrophy |
Providing a lasting solution |
|
Hemophilia |
Providing the gene for the missing clotting factor. |
|
Multiple Myeloma |
Target specific antigens on cancer cells. |
|
Neurodegenerative Diseases |
Delivering proteins that promote neuron survival |
Latest Trends of the Market ⬥︎ In July 2025, AscellaHealth, a global partner
delivering customizable solutions to support the specialty pharmaceutical
industry, highlights the value of its HUB partnership with Abeona Therapeutics
Inc. in the successful pre- and post-launch commercialization of ZEVASKYN, an
FDA-approved cell-based gene therapy. ⬥︎ In April 2025, INmune Bio Inc., a clinical-stage
biotechnology company targeting inflammation and immunology through the innate
immune system, partnered with the Cell and Gene Therapy Catapult to establish
large-scale, commercial-ready manufacturing for its cell therapy platforms. CGT
Catapult is an independent technology and innovation organization specializing
in the advancement of the cell and gene therapy industry. It was established by
and works in partnership with Innovate UK. Recent Advancements in CGT Tools and
Techniques: Market’s Largest Potential Recent advancements in new cutting-edge
tools and technologies for high-throughput, precise, and effective genome
editing for medical care claims. These advancements comprise new nucleic acid
formats and viral biosynthesis processes, non-viral gene therapy, cell
therapies, microfluidics or encapsulation-driven cell culture, in vivo
cell therapy, advanced GMP cell sorting, non-chromatographic separations, and
novel optical methods for nanoscale. CGT provides the advanced strength of novel
curative treatments for patients with excessive healthcare requirements in
immune conditions, hereditary diseases, oncology,
and other therapeutic areas. This rapid renovative advancement of the
therapeutic armamentarium is associated with current manufacturing to deliver
these sophisticated therapies. ⬥︎ For Instance, In September 2025,
INmune Bio Inc., a clinical-stage inflammation and immunology company focused
on developing treatments that harness the patient’s innate immune system to
fight disease, announced the successful completion of its first full-scale
pilot commercial manufacturing run of CORDStrom, an off-the-shelf, allogeneic,
umbilical cord tissue-derived mesenchymal stromal cell therapy for the
treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB). Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/7720 Cell and Gene Therapy Market Report
Scope Report Attribute Details Market Size in 2025 USD 25.89 Billion Market Size by 2034 USD 119.30 Billion Growth Rate From 2025 to 2034 CAGR of 18.5% Base Year 2024 Forecast Period 2025 to 2034 Segments Covered By Therapy Type, By Vector Type, By Application, By End User, By
Delivery Mode, By Industry Vertical, By Region Market Analysis (Terms Used) Value (US$ Million/Billion) or (Volume/Units) Report Coverage Revenue forecast, company ranking, competitive landscape, growth
factors, and trends Key Companies Profiled Novartis AG, Gilead Sciences (Kite Pharma), Bristol
Myers Squibb, Bluebird Bio, Orchard Therapeutics, Spark
Therapeutics (Roche), uniQure, CRISPR
Therapeutics, Editas Medicine, Intellia Therapeutics, Regenxbio, Sangamo Therapeutics, Fate
Therapeutics, Adaptimmune Therapeutics, Poseida Therapeutics, Mustang
Bio, Iovance Biotherapeutics, Pfizer Inc. (gene therapy
division), Bayer AG (cell & gene therapy investments), Takeda
Pharmaceutical
Regional Insights North America led the viral vector and
plasmid DNA
manufacturing market in 2024, due to the presence of an advanced
R&D ecosystem, such as strong pharmaceutical and industry leaders like
Biogen, Novartis, and Pfizer, which heavily invest in R&D. Increasing
government support to the healthcare sector, which approves fast clinical
therapies, drives the growth of the market. ⬥︎ For Instance, In September 2025,
ProteoNic, a leader in premium vector technology for biologics and cell and
gene therapy, announced the launch of its novel 2G UNic transposon platform,
available for licensing alongside the expansion of 2G UNic compatibility across
transposon systems used by biopharma customers. The U.S. is a leader in scientific and
technological services, with the presence of top research institutes such as
NIH, Harvard, and MIT. Increasing funding from the public sector, like BARDA
and ARPA-H, for cellular and genetic therapies, contributes to the growth of
the market. ⬥︎ For Instance, In September 2025,
Myrtelle Inc., a pioneering clinical-stage gene therapy company developing
transformative treatments for neurodegenerative diseases, announced the
publication of interim results from its Phase 1/2 clinical
trial of rAAV-Olig001-ASPA (MYR-101) in Nature Medicine. Why is the Asia Pacific the
fastest-growing Market? Asia Pacific is the fastest growing market
as the presence of a supportive government environment, like the FDA, approves
gene therapies rapidly than other regulatory bodies, allowing fast
commercialization. Huge network of specialty hospitals and different centers of
cell and gene therapy. Increasing demand for innovative treatment options, such
as advanced cell therapy, contributes to the growth of the market. ⬥︎ For Instance, In June 2025,
Singapore-based company Nuevocor received clearance from the FDA for an IND
application for NVC-001, an AAV vector-based gene therapy intended to treat
LMNA DCM. In light of the IND clearance, the company intends to go forward with
plans for a first-in-human phase 1/2 ascending-dose clinical trial. Nuevocor
expects to launch the 52-week trial, which will be open to adult patients,
early next year. Participants in the trial will receive a 1-time dose of the
gene therapy product via intravenous infusion. Cell and Gene Therapy Market, By Region,
2021-2024 (USD Million) By Region 2021 2022 2023 2024 North America 2,724.8 3,067.2 3,461.9 3,915.1 Europe 1,315.8 1,493.4 1,699.7 1,938.5 Asia Pacific 952.3 1,109.7 1,295.9 1,515.3 South America 95.9 105.5 116.3 128.3 Middle East & Africa 210.4 234.4 261.9 293.1
Cell and Gene Therapy Market
Segmentation Analysis: By Therapy Type Analysis: The cell therapy
segment dominates in the cell and gene therapy market, as these therapies are
used in patients to repair and regenerate tissues that are damaged or affected
by disease. It lessens patients' immune rejection challenges while endorsing
long-term curative processes. Prominent applications of cell therapies comprise
treating tumors, autoimmune disorders, UTI problems, and infectious diseases,
transforming damaged cartilage in joints, fixing spinal cord injuries, boosting
a weakened immune system, and supporting patients with brain-related disorders. On the other hand, the gene
therapy (in vivo) segment is the fastest-growing segment in the market,
as CGT offers advanced treatments for diseases that presently don’t have any
other options. Many gene therapies are single treatments, rather than
continuing medication that has to be taken. This therapy manages the cause of
the illness, not just the symptoms. Receiving gene therapy previously in the
course of a disease prevents the disease from causing severe damage. Cell and Gene Therapy Market By Therapy
Type, 2021-2024 (USD Million) By Therapy Type 2021 2022 2023 2024 Cell Therapy 3,330.1 3,766.3 4,271.5 4,854.2 Gene Therapy 1,969.1 2,244.0 2,564.2 2,936.0
By Vector Type Analysis: The viral vectors segment is dominating the
market in 2024, as it shows huge strength for cell and gene delivery to treat
various types of diseases such as cancer, metabolic diseases, heart defects,
and neurodegenerative disorders. Vectors have been engineered based on
adenoviruses, herpes simplex viruses, adeno-associated viruses, alphaviruses,
retroviruses, and lentiviruses. On the other hand, the non-viral vectors
segment is the fastest-growing segment in the market, as it ensures reduced
pathogenicity, reduced expenses, and simple production. Non-viral vectors offer
a greater safety advantage than viral vector approaches. Increasing
applications of non-viral vectors due to their bio-safety, including lower
immunotoxicity than the viral vector. Cell and Gene Therapy Market, By Vector
Type (Gene Delivery Method) 2021-2024 (USD Million) By Vector Type (Gene Delivery Method) 2021 2022 2023 2024 Viral Vectors 3,891.6 4,395.2 4,977.5 5,648.3 Adeno-Associated
Virus (AAV) 1,692.1 1,913.2 2,169.1 2,464.2 Lentivirus 1,162.6 1,307.9 1,475.3 1,667.4 Retrovirus 387.0 430.9 480.9 537.8 Herpes Simplex Virus
(HSV) 158.6 183.7 213.3 248.0 Adenovirus 294.4 344.2 403.0 472.4 Others 196.9 215.3 235.8 258.5 Non-Viral Vectors 1,407.6 1,615.1 1,858.2 2,142.0 Lipid Nanoparticles
(LNPs) 513.4 591.9 684.2 792.4 Naked DNA/RNA
Plasmids 353.5 402.6 459.7 525.8 Electroporation 205.8 235.8 270.9 311.8 Gene gun /
microinjection 89.6 101.7 115.8 132.1 CRISPR-Cas Delivery
Systems (non-viral) 166.5 194.1 226.7 265.2 Others 78.7 89.1 101.0 114.7
By Applications Analysis: The oncology segment dominated the market
in 2024, as CGT allows targeted interventions, ranging from the alteration of
faulty genes to the improvement of immune responses against cancer, therefore
providing novel opportunities for treating both solid and hematologic
malignancies. CGT tailored to a patient’s targeted genetic profile and the
genetic mutations driving their cancer. This personalized strategy increases
the likelihood of management success and reduces adverse effects. On the other hand, the rare and genetic
disorders segment is the fastest growing segment in the market, as gene therapy
rapidly provides a cure, fixing the genetic fault and freeing patients from
lifelong disease and the requirement for symptomatic or disease-modifying
treatment. Gene therapy is predominantly helpful because major rare and genetic
diseases are caused by a single genetic mutation. By fixing or compensating for
this mutation, gene therapy has the strength to offer a long-term, potentially
curative treatment. By End User Analysis: The pharmaceutical
and biotechnology segment dominated the market in 2024, as CGT provides
efficient therapy and treatments where none have been viable with outdated
pharma and small-molecule drugs. These therapies are significant to biopharma
because of the novel, targeted strategies they bring to cancer treatments.
Pharmaceutical organizations are harnessing cell and gene therapy to address
complicated healthcare conditions and advance therapeutic choices. On the other hand, the CROs & CDMOs
segment is the fastest-growing segment in the market, as CROs and CDMOs play a
significant role in progressing the growing pipeline of cell and gene products.
Researchers utilize the advantages of these strategic partnerships for
assurance of their projects. These organizations accelerate their healthcare
and commercial programs, and major biotech companies are choosing to work with
contract development and manufacturing organizations (CDMOs). ⬥︎ For Instance, In April 2025, AGC
Biologics Launched a New Dedicated Cell and Gene Business Division. The new
Cell and Gene Technologies Division will focus on elevating existing AGC
Biologics capabilities and supporting developers in need of capacity,
scientific capabilities, and technically qualified cell and gene CDMO
operators. Cell and Gene Therapy Market By End Use,
2021-2024 (USD Million) By End Use 2021 2022 2023 2024 Hospitals and
Specialty Clinics (administering therapies) 4,141.0 4,676.4 5,295.5 6,008.5 Government/Public
Health Bodies (NIH, EMA programs) 708.6 817.5 945.6 1,095.7 Others 449.6 516.4 594.6 686.0
By Delivery Mode Analysis: The ex vivo administration segment
dominated the market in 2024, as ex vivo therapies provide the capability to
exercise supplementary precise control over the process of producing or
manipulating genetic material. Ex vivo gene therapies are mostly utilized to
treat blood disorders and diseases, ranging from leukemia and lymphoma to
sickle cell disease (SCD) in children. On the other hand, the in vivo
administration segment is the fastest-growing segment in the market, as this
therapy involves directly introducing therapeutic genes into the patient’s
body. Innovative genetic material is first created in a laboratory by
scientists and then delivered to the patient’s cells directly, typically by
infusing it into the blood or injecting it directly into an intended organ. The
in vivo gene therapy process also includes CRISPR-Cas9
therapy, RNA Interference (RNAi), naked DNA injection, and more. By Industry Vertical Analysis: The biotechnology segment dominated the
market in 2024, as cell therapies show a major border and paradigm shift in biotechnology.
In contrast to conventional therapeutic modalities, cell therapies exist and
dynamically respond to biological cues to combat malignancies, restore impaired
tissues, regenerate tissues or lost biological functions, or increase the
body's own capability to defend against disease. On the other hand, the pharmaceuticals
segment is the fastest-growing segment in the market, as CGT has a lower cost,
quick supply of manufactured products in response to clinical demand, and
optimal organization and transport of cellular material. With progressive
engineering processes, scientists improve these cells to recognize better and
attack cancer. Cell and Gene Therapy Market Top
Companies • Gilead Sciences (Kite Pharma) • Bluebird Bio • Orchard Therapeutics • Spark Therapeutics (Roche) • uniQure • CRISPR Therapeutics • Intellia Therapeutics • Regenxbio • Sangamo Therapeutics • Adaptimmune Therapeutics • Poseida Therapeutics • Mustang Bio • Iovance Biotherapeutics • Pfizer
Inc. (gene therapy division) • Bayer
AG (cell & gene therapy investments) • Takeda Pharmaceutical What is Going Around the Globe? 🔹 In May 2025, through strategic acquisitions made by New York–based
investment firm Altaris, Minaris Regenerative Medicine and the U.S. and U.K.
operations of WuXi Advanced Therapies have been combined to form Minaris
Advanced Therapies, a global cell therapy CDMO and testing partner. The
company is headquartered in Philadelphia, Pennsylvania. 🔹 In April 2025, Artis officially emerged from stealth, marking its
launch as a San Diego-based cell and gene therapy CDMO with the acquisition of
personalized medicine compatriot Landmark Bio. 🔹 In October 2024, McKesson Corporation announced the launch of
InspiroGeneTM by McKesson, a dedicated business focused solely on supporting
the commercialization of cell and gene therapies (CGTs). With a scalable,
flexible suite of services and an experienced leadership team, InspiroGene
enables manufacturers, payers, and providers to navigate the complex CGT
commercialization landscape to ensure patients can access the life-changing
treatments they need. You can place an order or ask any
questions, please feel free to contact at sales@novaoneadvisor.com |
+1 804 441 9344 Related Report – 🔸Cell And Gene Therapy CDMO Market - https://www.novaoneadvisor.com/report/cell-and-gene-therapy-cdmo-market 🔸Advanced Therapy Medicinal Products CDMO Market- https://www.novaoneadvisor.com/report/advanced-therapy-medicinal-products-cdmo-market 🔸Oncology Clinical Trials Market- https://www.novaoneadvisor.com/report/oncology-clinical-trials-market 🔸Cell And Gene Therapy Manufacturing Market- https://www.novaoneadvisor.com/report/cell-and-gene-therapy-manufacturing-market 🔸U.S. Cell Therapy Market- https://www.novaoneadvisor.com/report/us-cell-therapy-market 🔸RNA Therapy Clinical Trials Market - https://www.novaoneadvisor.com/report/rna-therapy-clinical-trials-market 🔸Cell & Gene Therapy Contract Research Organizations Market- https://www.novaoneadvisor.com/report/cell-and-gene-therapy-contract-research-organizations-market Segments Covered in the Report This report forecasts revenue growth at country
levels and provides an analysis of the latest industry trends in each of the
sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has
segmented the Cell and Gene Therapy market. By Therapy Type • Cell Therapy o Autologous Cell
Therapy o Allogeneic Cell
Therapy o Stem Cell
Therapy (MSC, HSC, iPSC) o T-cell Therapy
(CAR-T, TCR-T, NK cells) o Dendritic Cell
Therapy o Others • Gene Therapy o Gene Addition /
Replacement o Gene Silencing
(RNAi, antisense) o Genome Editing
(CRISPR/Cas, TALEN, ZFN) o Ex Vivo Gene
Therapy o In Vivo Gene
Therapy o Others By Vector Type • Viral Vectors o Adeno-associated
Virus (AAV) o Lentivirus o Retrovirus o Adenovirus o Herpes Simplex
Virus (HSV) o Others • Non-Viral Vectors o Lipid Nanoparticles
(LNPs) o Electroporation o Naked DNA / RNA o Physical &
Chemical Methods (polymeric nanoparticles, nanocarriers) o Others By Application • Oncology (hematological malignancies,
solid tumors) • Rare & Genetic Disorders (hemophilia,
SMA, DMD) • Cardiovascular Diseases • Neurological Disorders • Ophthalmic Disorders • Infectious Diseases • Musculoskeletal Disorders • Others By End User • Hospitals & Clinics • Academic & Research Institutes • Pharmaceutical & Biotechnology
Companies • Contract Research & Manufacturing
Organizations (CROs/CDMOs) • Others By Delivery Mode • In Vivo Administration • Ex Vivo Administration By Industry Vertical • Pharmaceuticals • Biotechnology • Healthcare & Diagnostics • Research & Academia • Others By Region • North America • Europe • Asia-Pacific • Latin America • Middle East & Africa (MEA) Immediate Delivery Available | Buy This
Premium Research https://www.novaoneadvisor.com/report/checkout/7720 About-Us Nova One Advisor is a global leader
in market intelligence and strategic consulting, committed to delivering deep,
data-driven insights that power innovation and transformation across
industries. With a sharp focus on the evolving landscape of life sciences, we
specialize in navigating the complexities of cell and gene therapy, drug
development, and the oncology market, enabling our clients to lead in some of
the most revolutionary and high-impact areas of healthcare. Our expertise spans the entire
biotech and pharmaceutical value chain, empowering startups, global
enterprises, investors, and research institutions that are pioneering the next
generation of therapies in regenerative medicine, oncology, and precision
medicine. Web: https://www.novaoneadvisor.com/ Contact Us USA: +1 804 420 9370 Email: sales@novaoneadvisor.com For Latest Update
Follow Us: LinkedIn

