Know Labs, Inc. (OTCQB: KNWN), an emerging leader in non-invasive medical diagnostics, today announced that new independent test data have confirmed the efficacy of a light bulb produced by its wholly owned subsidiary Particle, Inc., in inactivating multiple variants of SARS-CoV-2, the virus that causes COVID-19.
Company Provides Update on Particle Subsidiary
SEATTLE--(BUSINESS WIRE)-- Know Labs, Inc. (OTCQB: KNWN), an emerging leader in non-invasive medical diagnostics, today announced that new independent test data have confirmed the efficacy of a light bulb produced by its wholly owned subsidiary Particle, Inc., in inactivating multiple variants of SARS-CoV-2, the virus that causes COVID-19. The data are based on tests performed by Texas Biomedical Research Institute (Texas Biomed).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211013005140/en/
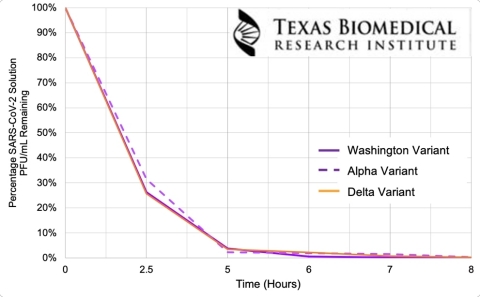
Test results reported by Texas Biomed demonstrate the Particle bulb’s efficacy in inactivating SARS-CoV-2 and its variants at the energy level equivalent to five feet distance from the light bulb. (Graphic: Business Wire)
Particle is an everyday light bulb that both illuminates and safely disinfects spaces where it is used, including homes and offices. Earlier this year, Know Labs confirmed that Particle bulbs were effective against the Washington SARS-CoV-2 strain. Multiple tests performed by Texas Biomed demonstrated a 95% reduction in the SARS-CoV-2 virus after 5 hours of exposure to the Particle bulb; a 99% reduction in 6 hours; and a 99.9% reduction in 8 hours. Texas Biomed recently conducted additional tests which confirmed that Particle bulbs were equally efficient against the Alpha and Delta variants.
“Texas Biomed is a world-renowned research institute specializing in infectious diseases. Having Particle bulbs tested by its team is an important step to confirm the bulb’s efficacy,” said Phil Bosua, Know Labs CEO and Particle bulb inventor. “We’ve seen other variants become a growing concern. Particle has the potential to help the world address this pandemic as well as assist with the control of other pathogens and viruses. We are very proud of what the Particle team has accomplished.”
Along with the Texas Biomed test results, the Company has received product and packaging samples from its contract manufacturer in Asia, a final step before commercial production can begin. With these milestones achieved and clear demand and need for a product like Particle, the Company is exploring potential strategic and channel partner opportunities.
“The Particle team has brought this important product very close to market in a short period of time,” Bosua added. “As Know Labs continues to focus on the Bio-RFID technology and the development of our non-invasive blood glucose monitoring devices, we are now actively seeking a partner to move Particle into the marketplace.”
For more information on Particle, please contact pr@particlebulb.com.
About Texas Biomed
Texas Biomed is one of the world’s leading independent biomedical research institutions dedicated to eradicating infection and advancing health worldwide through innovative biomedical research. Texas Biomed partners with researchers and institutions around the world to develop vaccines and therapeutics against viral pathogens causing AIDS, hepatitis, hemorrhagic fever, tuberculosis and parasitic diseases responsible for malaria and schistosomiasis disease. The Institute has programs in host-pathogen interaction, disease intervention and prevention, and population health to understand the links between infectious diseases and other diseases such as aging, cardiovascular disease, diabetes and obesity. For more information on Texas Biomed, go to www.TxBiomed.org.
About Know Labs, Inc.
Know Labs, Inc. is a public company whose shares trade under the stock symbol “KNWN.” The Company’s technology uses spectroscopy to direct electromagnetic energy through a substance or material to capture a unique molecular signature. The Company refers to its technology as Bio-RFID™. The Bio-RFID technology can be integrated into a variety of wearable, mobile, or bench-top form factors. This patented and patent-pending technology makes it possible to effectively conduct analyses that could only previously be performed by invasive and/or expensive and time-consuming lab-based tests. The first application of our Bio-RFID technology will be in a product marketed as a glucose monitor. It will provide the user with real-time information on their blood glucose levels. This product will require U.S. Food and Drug Administration approval prior to its introduction to the market.
Safe Harbor Statement
This release contains statements that constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements appear in a number of places in this release and include all statements that are not statements of historical fact regarding the intent, belief or current expectations of Know Labs, Inc., its directors or its officers with respect to, among other things: (i) financing plans; (ii) trends affecting its financial condition or results of operations; (iii) growth strategy and operating strategy; and (iv) performance of products. You can identify these statements by the use of the words “may,” “will,” “could,” “should,” “would,” “plans,” “expects,” “anticipates,” “continue,” “estimate,” “project,” “intend,” “likely,” “forecast,” “probable,” “potential,” and similar expressions and variations thereof are intended to identify forward-looking statements. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, many of which are beyond Know Labs, Inc.’s ability to control, and actual results may differ materially from those projected in the forward-looking statements as a result of various factors. These risks and uncertainties also include such additional risk factors as are discussed in the Company’s filings with the U.S. Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended September 30, 2020, Forms 10-Q and 8-K, and in other filings we make with the Securities and Exchange Commission from time to time. These documents are available on the SEC Filings section of the Investor Relations section of our website at www.knowlabs.co. The Company cautions readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made. The Company undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date on which such statement is made.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211013005140/en/
Source: Know Labs, Inc.







