Naviswiss, a Swiss-based medical technology company receives clearance from the FDA to market Naviplan in the United States.
|
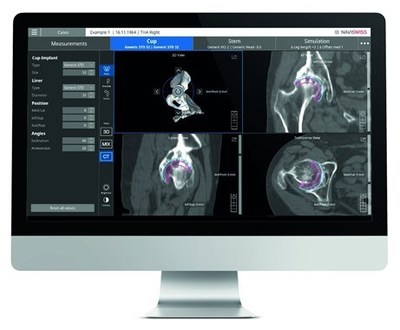
DENVER, Oct. 21, 2021 /PRNewswire/ -- Naviswiss, a Swiss-based medical technology company receives clearance from the FDA to market Naviplan in the United States. Naviplan is a digital pre-operative planning application enabling orthopedic surgeons to perform navigated CT-based total hip replacement surgery. This has the potential to improve accuracy, predictability and provide seamless documentation of the outcome. Naviswiss, the technology leader in miniaturized surgical navigation solutions, supports orthopedic surgeons in accurately positioning hip replacement implants. The Naviplan hip application is CT-based and assists the surgeon in the optimal positioning of the joint implants, automatic 3D segmentation and advanced image processing. Naviplan outputs the pre-operative plan into the Naviswiss navigation platform for accurate surgical execution. "Naviplan and CT-based Navigation are an important addition to the Naviswiss portfolio and completes our offering for navigated hip replacement," said Jan Stifter, Naviswiss CEO. "We now have two patient-specific options where the surgeon determines the best application for the procedure. CT-based Navigation may be needed in difficult deformity cases while kinematic registration may be preferred in more traditional surgeries. The surgeon can rely on highly accurate guidance in placing the acetabular components." Naviswiss is releasing the CT-based Navigation and Naviplan to orthopedic care centers in the United States over the course of the fourth quarter 2021. About Naviswiss
SOURCE Naviswiss |