SAN DIEGO, CA--(Marketwire - November 26, 2012) -
| Highlighted Links |
Naviscan Company Website |
Naviscan Products |
Michael O'Connor, PhD, Mayo Clinic, will present Technical Developments in Molecular Imaging of the Breast. In this educational session, Dr. O'Connor will review PEM, MBI and BSGI starting with a discussion of detector configurations and reviewing current applications of molecular breast imaging in the clinical care cycle. The session will conclude with a discussion of potential future applications of this technology in understanding breast disease.
Lawrence MacDonald, PhD, University of Washington, Seattle will present Physics and Practical Aspects of Breast PET (PEM). In this physics session, Dr. MacDonald reviews the technological developments in dedicated breast PET imaging technologies including the factors that affect quantitation of breast lesions. New exciting results in FDG dose reductions and new areas of future research will be summarized.
Wendie A. Berg, MD, PhD, University of Pittsburgh School of Medicine, will present Practical Aspects and Clinical Status of Breast PET (PEM). Dr. Berg will review the applications of dedicated high resolution PET devices (PEM) and describe the interpretive criteria in this clinical session. New results in FDG dose reduction and future research directions will be discussed.
"These sessions demonstrate the expanding role and utility of PEM in the diagnosis of breast cancer," stated Paul J. Mirabella, CEO, Naviscan, Inc. "Because of the continued research of these and other esteemed clinicians utilizing PEM, the practical aspects of breast cancer diagnosis and treatment are ever improving."
About Naviscan, Inc.
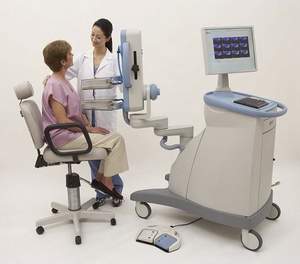
Naviscan, founded in 1995, develops and markets compact, high-resolution PET scanners intended to provide organ-specific molecular imaging and guide radiological and surgical procedures. The Naviscan PET scanner is currently installed and available in breast and imaging centers throughout the world with global distribution in 34 countries. The Company is headquartered in San Diego, California and is the first to obtain FDA-clearance and CE Mark for a high-resolution PET scanner designed to image small body parts and for breast biopsy image guidance. For more information, visit www.naviscan.com.
Contact Information
Joleen Schultz
Naviscan Marketing Communications
321 Medical Launch
Tel: +1.858.455.5500
Email Contact

 Digg this
Digg this Bookmark with del.icio.us
Bookmark with del.icio.us Add to Newsvine
Add to Newsvine


