Massachusetts
Last month, biopharmas let go or projected they would let go of less than 500 people combined, based on BioSpace estimates, down almost 1,000 from January 2025. Still, competition for open jobs remains strong, with employed and unemployed biotech and pharma professionals eyeing their next roles.
Looking for a biopharma job in Boston? Check out the BioSpace list of nine companies hiring life sciences professionals like you.
Looking for an IT job? From data engineer to information security, check out the BioSpace list of 10 companies hiring life sciences professionals like you.
Looking for a research and development job? Check out the BioSpace list of 12 companies hiring life sciences professionals like you.
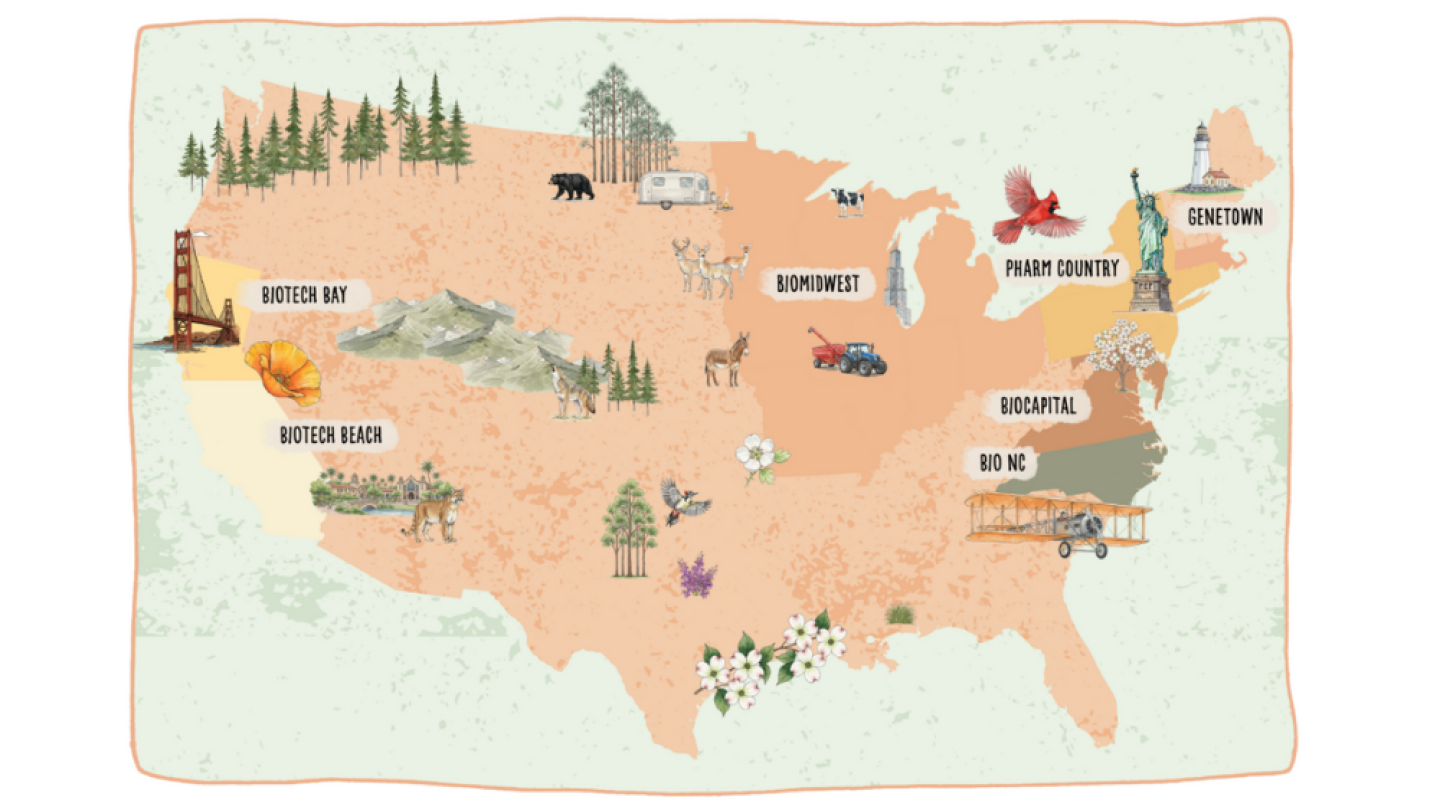
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
Less than six months after cutting 20% of its employees, Vedanta Biosciences has again laid off staff. According to one affected staffer, half of the Cambridge, Massachusetts–based biotech’s workforce is being cut while most of the rest are furloughed.
Looking for a manufacturing job? Check out the BioSpace list of 11 companies hiring life sciences professionals like you.
In 2025, made or projected biopharma workforce cuts affected about 42,700 employees, according to BioSpace tallies. BioSpace takes a deep dive into which companies and locations were impacted and speaks to experts about what to expect ahead—and why.
Biopharma professionals will probably find decreasing employment opportunities this month and next even as layoffs continue, based on BioSpace data. However, hundreds of open roles are expected this year in Massachusetts, and a job market turnaround could start late next year.
Nearly two dozen life sciences companies that were awarded Massachusetts tax incentives to create and retain about 1,000 combined jobs hit just 13% of that target in 2024. Ten awardees had reported layoffs last year, including Charles River Laboratories and Moderna.
PRESS RELEASES