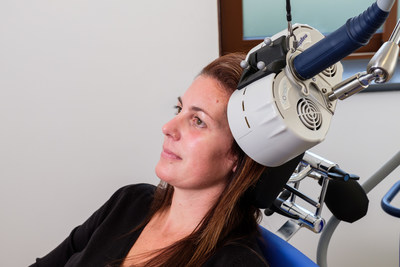
The Horizon ® system was designed to offer more versatility in one system, and more treatment options for patients.
|
EDEN PRAIRIE, Minnesota, April 4, 2019 /PRNewswire/ -- Magstim has received FDA clearance to include intermittent Theta Burst Stimulation (iTBS) as a treatment for Major Depressive Disorder with its Horizon ® TMS Therapy systems.
The Horizon ® system was designed to offer more versatility in one system, and more treatment options for patients. This clearance allows Magstim to market the 3-minute iTBS protocol. Clinicians have options to deliver treatments with the standard repetitive Transcranial Magnetic Stimulation (rTMS), which takes 37.5 minutes, accelerated TMS (19 minutes) and iTBS, which takes just three minutes per treatment. The Horizon® System was designed with the proprietary Energy Recovery System (ERS) that ensures consistency throughout the treatment with no pulse amplitude decay. The ability to offer a variety of treatment options with consistent "dosage" throughout a treatment is a unique distinction in the industry. Customers who already have a Magstim Horizon® Performance System will not need to purchase new equipment or upgrade to their system to deliver the iTBS protocol. The E-z Cool Coil, included in the Horizon® Performance package, has been specifically designed for iTBS, while still providing the option for standard rTMS protocols. Magstim, the pioneer in TMS, developed the first TMS technology over 30 years ago, and continues to be a leader in the field. With efficacy equal to that of standard rTMS, intermittent Theta Burst Stimulation is the newest TMS protocol that maximizes patient throughput. With a range of treatment protocols available in one system, Magstim gives practitioners the flexibility to tailor the best treatments for patients without the need to change equipment. In depressed patients, electrical activity in certain areas of the brain is reduced. The Horizon® Performance uses a focused electromagnetic coil to rapidly pulse a magnetic field to the targeted area of the brain. This induces a small electrical current which stimulates the targeted brain cells into activity, gradually increasing brain activity back to a normal level. Magstim's Group CEO Lothar Krinke said: "I am pleased that we can now market the full capabilities of the Horizon Performance platform. The versatility coupled with the proprietary Energy Recovery System gives us a distinct advantage in the market. We want to provide innovative systems that allow clinicians and researchers to customize and personalize treatment for patients, and this clearance is another step in that direction." iTBS is already available through Magstim's Horizon® systems in the UK and EU. Professor Alex O'Neill-Kerr is the clinical lead for The Centre for Neuromodulation at Berrywood Hospital in Northampton and a recognized expert in rTMS in the UK. He said: "Theta burst therapy is rapidly gaining popularity among TMS Centers in the UK based on evidence from elsewhere in the world, it is as effective as standard TMS. In the NHS, having the option of theta burst allows us to tailor the best treatment protocol for the patient. "The Centre for Neuromodulation is one of a multi-center NIHR funded clinical trial, the BRIGhTMIND study, evaluating standard rTMS vs navigated iTBS in depression. Recruitment has started and this trial will contribute to the evidence base for theta burst treatment in future particularly in the NHS." Please direct media enquiries to Jo Wallace at Yogi on +44-(0)2920-460-234 or jo.wallace@yogicomms.uk
SOURCE Magstim |