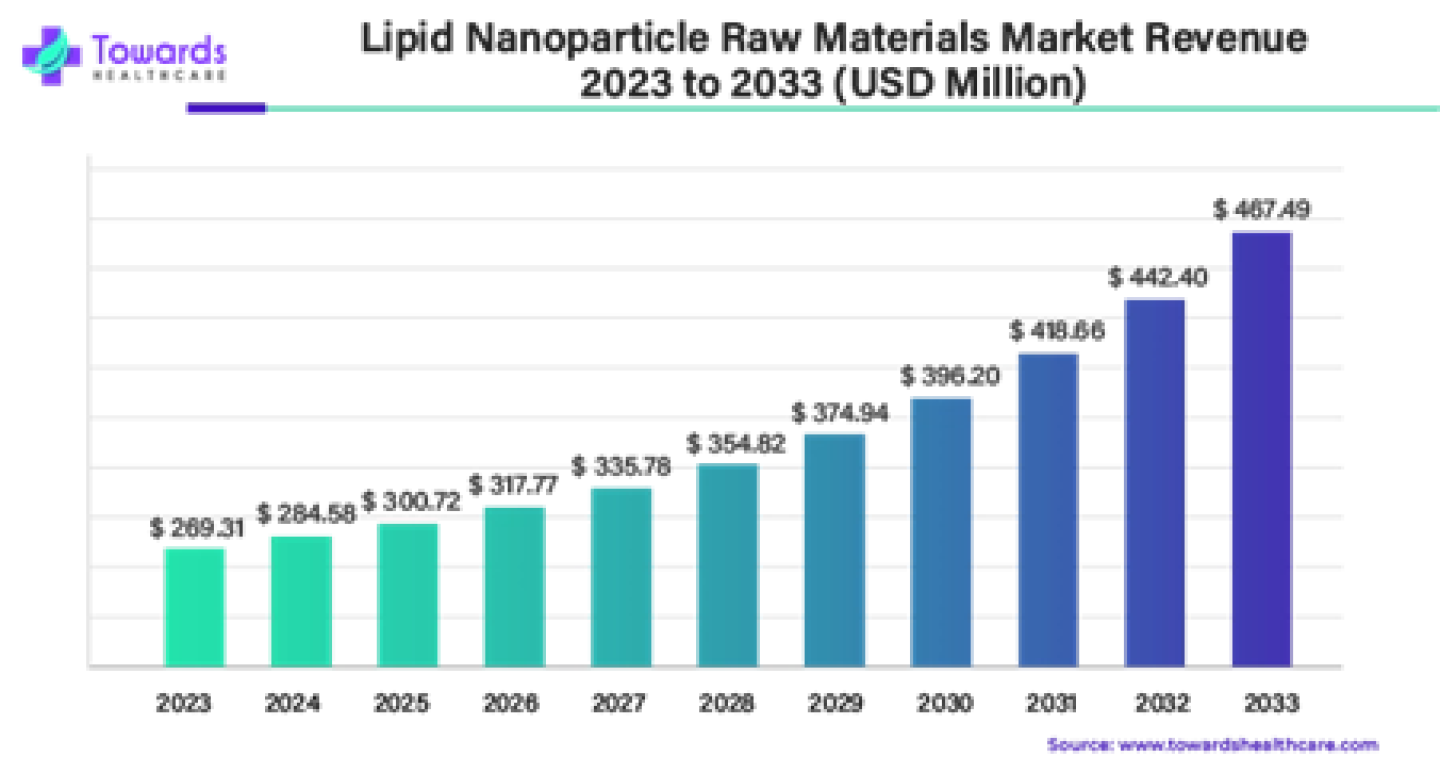
The global lipid nanoparticle raw materials market size was valued at USD 269.31 in 2023 and is expected to grow to USD 467.49 by 2033, with a CAGR of 5.67% during the forecast period (2024 – 2033).
Increasing demand for drugs that utilize lipid nanoparticles (LNPs) as delivery systems and advancements in technology are all driving growth in the lipid nanoparticle raw materials market.
Download a short version of this report @ https://www.towardshealthcare.com/personalized-scope/5168
Key Takeaways:
Lipid Nanoparticle Raw Materials Market at a Glance
Lipid nanoparticles are spherical vesicles made of ionizable lipids with a low or neutral pH that are used to carry drugs, genetic material, or imaging agents to specific cells or tissue in the body. The balanced pH of these particles reduces potential toxic effects compared to positively charged lipids like liposomes. LNPs usually contain helper lipids that promote cell binding along with polyethylene glycol that reduces opsonization by serum proteins and reticuloendothelial clearance (RES). These elements contribute to the enhanced drug stability, solubility, and efficacy of lipid nanoparticles in vivo.
There is significant growth in the demand for lipids, polymers, surfactants, and stabilizers due to a wide range of applications of lipid nanoparticles in pharmaceutical drugs, diagnostics, food, cosmetics, and agriculture. The pharmaceutical and biotechnology sector in particular has seen a significant demand in the last five years for LNPs in drug delivery. There is also a rise in funding for research and development of lipid nanoparticles due to the recent prevalence of infectious diseases and chronic conditions like cancers and autoimmune disorders, furthering the need for effective treatment solutions.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Top companies in the Lipid Nanoparticle Raw Materials Market
Factors such as climate change, rapid urbanization, and global trade and mobility are increasing the risk of infectious diseases globally. Climate change is forcing pathogens to adapt and create new threats of vector-borne infections, which have the potential to rapidly reach new locations. An estimated 75% of infectious diseases originate in animals, including SARS, MERS, dengue, Ebola, Zika, influenza, and COVID-19.
Changing land-use patterns are increasing frequent interactions between people and once-remote animals. The infectious diseases segment in the LNP market is growing at a rapid rate due to the demand for new medicine delivery mechanisms. LNPs show promise in preventing mRNA from being broken down in the body, increasing treatment efficiency. In addition, lipid nanoparticles are also being tapped for developing treatments for infectious diseases like HIV and influenza. This has further contributed to the growth in the lipid nanoparticle raw materials market.
A rapidly aging population and rising risk factors associated with socioeconomic development, obesity, alcohol, tobacco consumption, and air pollution are causing a rise in global incidences of cancer. The WHO predicts that over 35 million people will be affected by cancer by 2050. The rise in cancer and lung disease cases is also fueling medical research and the development of new drugs and treatment protocols. Innovation in LNPs, especially in the gene editing space for lung disease and cystic fibrosis, is driving attention and funding.
Developments in siRNA encapsulated lipid nanoparticle (LNP) in vivo delivery systems are proving to be highly efficient at targeting respiratory syncytial virus (RSV) lung infections in vivo. LNPs offer promising new drug-delivery platforms by being able to encapsulate a variety of medications. All these factors are driving demand for raw materials used in the production of lipid nanoparticles.
Customize this study as per your requirement @ https://www.towardshealthcare.com/customization/5168
Increasing Adoption of LNPs in mRNA and Gene Editing Technology
LNPs are gaining popularity in delivering therapeutics, including those based on nucleic acids and mRNA. Advancements in mRNA technologies have changed the way new pharmaceuticals are being developed on a global scale. mRNA technology was widely used in the creation of COVID-19 vaccinations and shows promise in the development of more effective antigen therapies.
The development of new, lipid nanoparticle-based CRISPR-Cas9 carrier systems is also a game-changer in gene editing technology. Certain LNPs like Polyethylene glycol-phospholipid-modified cationic lipid nanoparticles show significant promise for efficient CRISPR-Cas9 delivery as a gene editing instrument.
Burst Release System Restrains the Lipid Nanoparticle Raw Materials Market Growth
While research and development in LNPs is showing promising results for more sophisticated drug delivery mechanisms, a major problem encountered with lipid nanoparticles is the burst release observed with these systems. Production-related factors such as temperature variations, changes in the lipid matrix, and surfactant makeup can change the release patterns of medication housed in the lipid nanoparticles.
Interactions with the reticuloendothelial system (RES) are also a major hurdle for the administration of LNPs. The administered nanoparticle prompts a biological response from the body, resulting in the formation of protein coronas. Certain elements (called opsonins) in the corona can vary the uptake of the coated materials by cells of the RES. The “molecular signature” left behind by opsonins in the corona can affect the routes of particle internalization. The routes taken can affect the medication’s volume of distribution, organ disposition, and rate of clearance from the bloodstream.
Complex Processes in Lipid Nanoparticle Productio
The production of lipid nanoparticles is a major challenge in the growth of the raw material market, lowering overall demand. Lipid dispersions have limited transdermal and hydrophilic drug delivery capacity. LNPs are also subject to polymorphic change, lowering the effectiveness of molecule delivery. The toxicity of lipid nanoparticles in certain cell types (e.g., retinal cells) has not yet been researched enough, raising questions about their use in the delivery mechanisms of certain medications. The high costs of lipid nanoparticle production also hinder the widespread adoption of the technology just yet.
Gene Therapy Creates Opportunity for the Lipid Nanoparticle Raw Materials Market
The application of LNPs in delivering gene editing therapies like CRISPR-Cas 9 shows promise in the treatment of genetic disorders and conditions like cystic fibrosis, sickle cell anemia, and muscular dystrophy. The use of LNPs is also being researched for deliveries of RNA-based therapies to treat neurological conditions such as Parkinson’s, amyotrophic lateral sclerosis (ALS), and Alzheimer’s. LNPs are proving instrumental in developing treatments for rare diseases where tailored therapies are necessary.
Lipid nanoparticles have gained traction in gene therapy as non-viral delivery systems demonstrating efficiency in delivering small-interfering RNAs (siRNAs) and DNA including cationic lipids, cell-penetrating peptides, liposomes, and cationic polymers. Cationic SLNs show promise for use in gene delivery due to the electrostatic interactions between the positive charges of the lipid and the negative charges of the DNA. These interactions cause the formation of lipoplexes which protects the DNA and directs it smoothly towards target cells.
Increased R&D and Government Funding in Lipid Nanoparticle Research
The promise shown by LNPs in both the pharmaceutical and biotechnology industries has led to increased spending on R&D. Increasing government funding is playing a crucial towards innovation in the space. Government funding initiatives by the National Institutes of Health (NIH) in North America have crossed over USD 228.4 million in the past three years. The U.S. Biomedical Advanced Research and Development Authority (BARDA) has also directed USD 150 million in funding for LNPs in 2022. Massachusetts-based Hopewell Therapeutics has also acquired $25 million in seed funding for developing LNPs for delivering nucleic acid cargos to treat lung diseases.
In Europe, AlgiPharma has received more than USD 46 million for research and development in LNPs in the form of grants from the EU’s 7th framework and Eurostars programs. The long-term promise showed by LNPs as effective drug delivery platforms is contributing to significant interest in the technology and attracting.
North America Leads the Lipid Nanoparticle Raw Materials Market
North America dominates in the global lipid nanoparticle raw materials market with a notable share. Several factors including significant research and development spending from the universities, biotechnology firms, and the government have helped the region dominate the market. Investment from the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services has also spurred advancements in lipid nanoparticle research. The global impact of Pfizer-BioNTech and Moderna's LNP-based mRNA COVID-19 vaccines have helped boost the industry significantly and generated much interest in lipid nanoparticle research.
Canada has also made a significant contribution to the expansion of the LNP raw material market. For instance, in April 2023, the government of Canada approved C$200 million in funding through development agencies for integrating AI in the development of agriculture and healthcare-based LNPs.
Asia-Pacific is the Fastest-Growing Region in the Lipid Nanoparticles Raw Materials Market
The Asia-Pacific houses the fastest-growing sector of the lipid nanoparticle raw materials market due to the growing pharmaceutical industry in India and China and increased government healthcare spending. A rise in incidences of infectious diseases and chronic conditions in the region has led to increased government budget allocations towards research and development of LNPs. In Asia, the number of cancer cases has gone up to 9.4 million, having doubled by 2019 in comparison to 1990. As more people get affected by such conditions, the demand for treatments, including those centered around lipid nanoparticles has gone up.
Government initiatives in South Korea in the past few years have focused on new drug delivery methods, including lipid nanoparticles, fostering a strong collaboration between the industry and academic research institutes. For instance, researchers in the Center for Nano Manufacturing and the Department of Nanoscience and Engineering at Inje University have focused on developing a solid lipid nanoparticle (SLN) system to enhance Curcumin bioavailability to utilize its anticancer properties.
By Product, the Kits Segment holds the Largest Share in Market
The kits segment was the largest in the market in 2023. Lipid nanoparticle kits can be used to create components for therapeutic uses that target specific cells and tissue types. Kits provide a way of continuously refining the manufacturing process to increase stability and payload delivery in lipid nanoparticle products. LNP kits are versatile with uses in cellular imaging, drug screening, and the creation of fluorescent probes and dyes, leading to a rise in their demand for academic and biotechnology research. The growth of the personalized medicine sector is contributing to the growth in the kit segment of the LNP market with the promise of increasingly customizable formulations.
The reagent segment is expected to be the fastest growing in the forecast period. Reagents enable researchers to establish a clinically relevant and scalable method for ex vivo gene delivery and editing as well as enable target validation. Reagents help in efficient knockout, and expression, and enhance cell viability, making them essential components of lipid nanoparticle research. Increasing investment in biotechnology-based R&D activities and the growing need for screening chronic diseases are contributing to the growth of this segment.
Browse More Insights of Towards Healthcare:
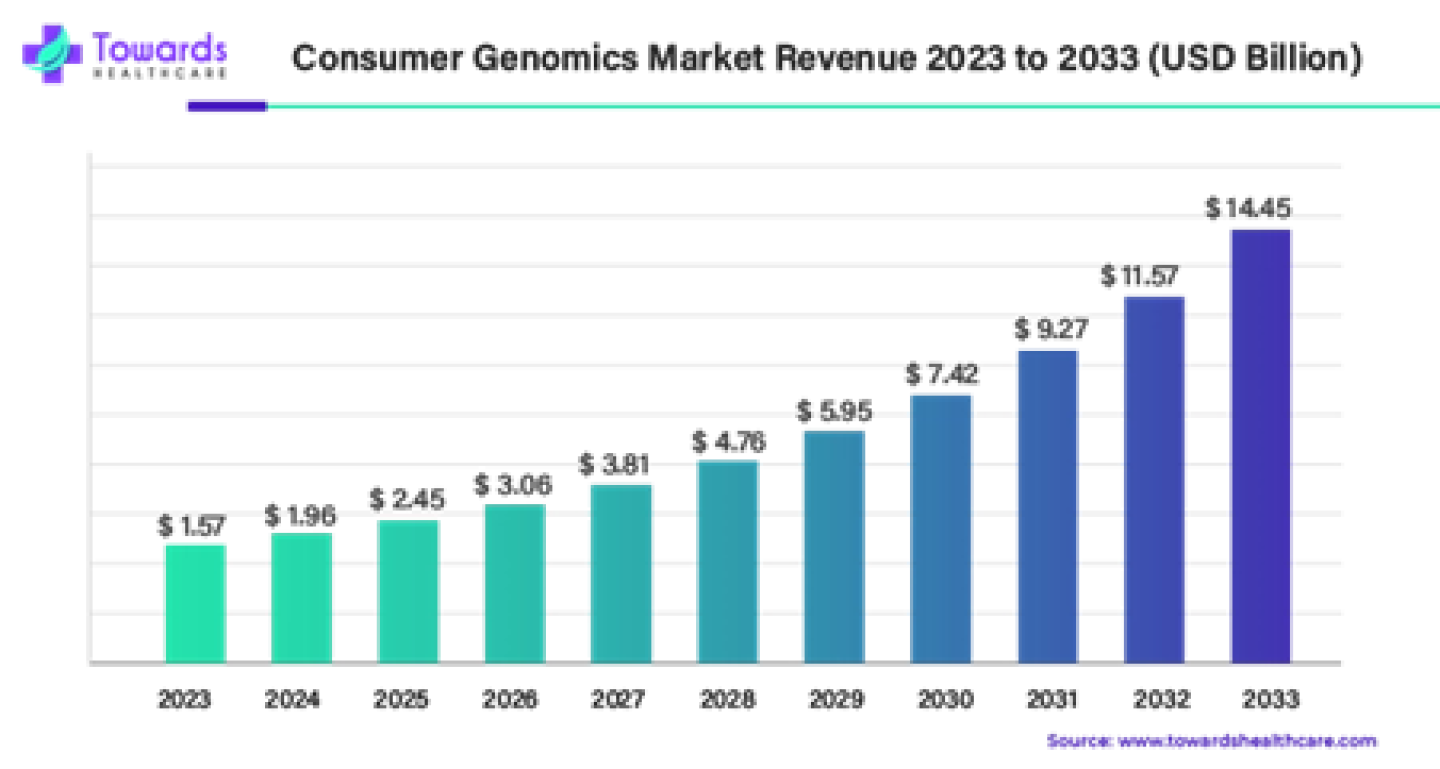
1. Consumer Genomics Market Size, Share and Trends Report
The global consumer genomics market size was estimated at US$ 1.57 billion in 2023 and is projected to grow to US$ 14.45 billion by 2033, rising at a compound annual growth rate (CAGR) of 24.85% from 2024 to 2033.
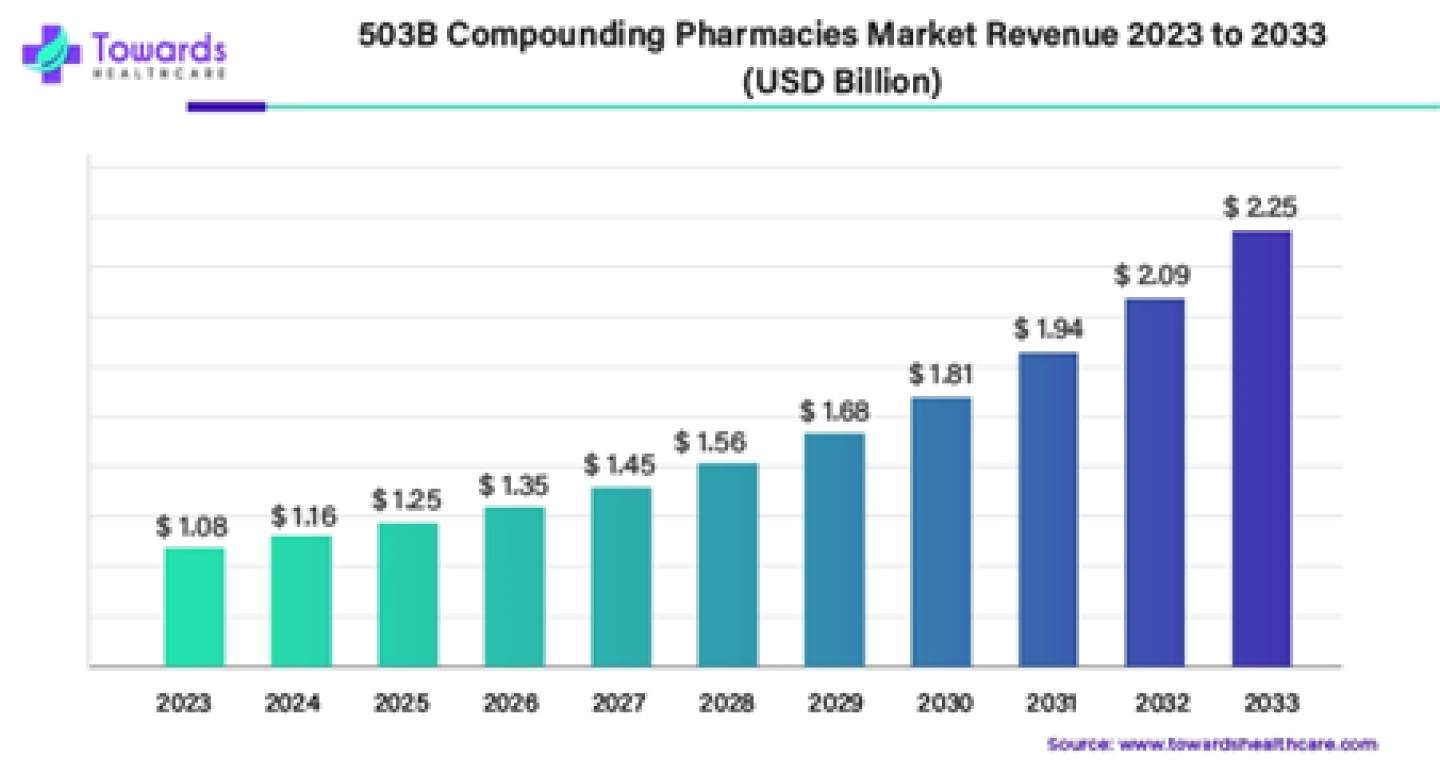
2. 503B Compounding Pharmacies Market Manufacturers Analysis Report
The global 503B compounding pharmacies market size was estimated at US$ 1.08 billion in 2023 and is projected to grow US$ 2.25 billion by 2033, rising at a compound annual growth rate (CAGR) of 7.63% from 2024 to 2033.
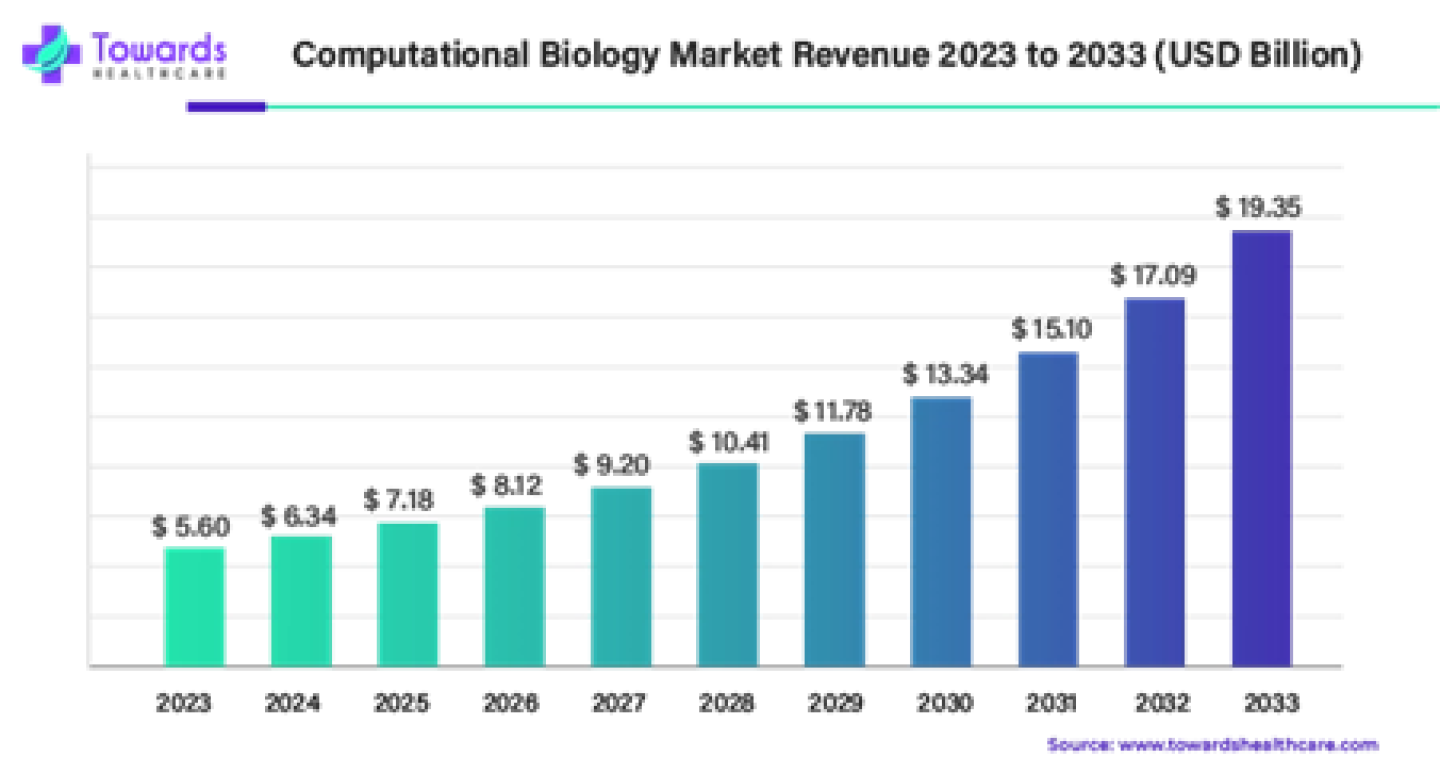
3. Computational Biology Market Size, Growth and Trends Report
The global computational biology market size was estimated at US$ 5.60 billion in 2023 and is projected to grow US$ 19.35 billion by 2033, rising at a compound annual growth rate (CAGR) of 13.20% from 2024 to 2033.
4. The global biomaterials market size was estimated at US$ 178.16 billion in 2023 and is projected to grow US$ 761.23 billion by 2033, rising at a compound annual growth rate (CAGR) of 15.63% from 2024 to 2033.
5. The global circulating tumor cells market size was estimated at US$ 11.49 billion in 2023 and is projected to grow US$ 41.27 billion by 2033, rising at a compound annual growth rate (CAGR) of 13.64% from 2024 to 2033.
6. The global gene synthesis market size was estimated at US$ 2.1 billion in 2023 and is projected to grow US$ 9.38 billion by 2033, rising at a compound annual growth rate (CAGR) of 16.14% from 2024 to 2033.
7. The global sequencing market size was estimated at US$ 15.59 billion in 2023 and is projected to grow US$ 115.85 billion by 2033, rising at a compound annual growth rate (CAGR) of 22.21% from 2024 to 2033.
8. The global retinal biologics market size was valued at US$ 23.18 billion in 2023 is expected to reach US$ 49.67 billion by 2033, at a compound annual growth rate (CAGR) of 7.92% from 2024 to 2033.
9. The cancer vaccines market was valued at US$ 10.21 billion in 2023 and is predicted to reach US$ 30.16 billion by the end of 2033, representing an impressive CAGR of 11.44% from 2024 to 2033.
10. The respiratory drugs market size is estimated to grow from 16.57 billion in 2023 to reach around USD 28.11 billion by 2032, registering a CAGR of 5.8% between 2024 and 2032.
Major Breakthroughs in Lipid Nanoparticle Raw Materials Market
Executive Summary
By Product
By Product vs. By Disease Indication
Executive Summary
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Browse our Brand-New Journals:
https://www.towardspackaging.com
https://www.towardsautomotive.com
https://www.precedenceresearch.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare
Get Our Freshly Printed Chronicle: https://www.healthcarewebwire.com
Increasing demand for drugs that utilize lipid nanoparticles (LNPs) as delivery systems and advancements in technology are all driving growth in the lipid nanoparticle raw materials market.
Download a short version of this report @ https://www.towardshealthcare.com/personalized-scope/5168
Key Takeaways:
- North America dominated the market with the largest revenue share of 41% in 2023.
- By product, the kits segment has held a major revenue share of 55% in 2023.
- By disease indication, the infectious diseases segment has contributed more than 47% of revenue share in 2023.
- By disease indication, the cancer segment is expected to expand at a soldi CAGR during the forecast period.
- By application, the therapeutics segment has generated more than 63% of revenue in 2023.
- By application, the research segment is estimated to grow at the fastest CAGR during the forecast period.
Lipid Nanoparticle Raw Materials Market at a Glance
Lipid nanoparticles are spherical vesicles made of ionizable lipids with a low or neutral pH that are used to carry drugs, genetic material, or imaging agents to specific cells or tissue in the body. The balanced pH of these particles reduces potential toxic effects compared to positively charged lipids like liposomes. LNPs usually contain helper lipids that promote cell binding along with polyethylene glycol that reduces opsonization by serum proteins and reticuloendothelial clearance (RES). These elements contribute to the enhanced drug stability, solubility, and efficacy of lipid nanoparticles in vivo.
There is significant growth in the demand for lipids, polymers, surfactants, and stabilizers due to a wide range of applications of lipid nanoparticles in pharmaceutical drugs, diagnostics, food, cosmetics, and agriculture. The pharmaceutical and biotechnology sector in particular has seen a significant demand in the last five years for LNPs in drug delivery. There is also a rise in funding for research and development of lipid nanoparticles due to the recent prevalence of infectious diseases and chronic conditions like cancers and autoimmune disorders, furthering the need for effective treatment solutions.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Top companies in the Lipid Nanoparticle Raw Materials Market
- Polysciences, Inc.
- NOF AMERICA CORPORATION
- Biopharma PEG Scientific Inc.
- Creative Biolabs
- CordenPharma International
- Tebubio
- Avanti Polar Lipids
- Hopewell Therapeutics
- Echelon Biosciences
- Merck KGaA
- BroadPharm
- Cytiva
- Nippon Fine Chemical
Factors such as climate change, rapid urbanization, and global trade and mobility are increasing the risk of infectious diseases globally. Climate change is forcing pathogens to adapt and create new threats of vector-borne infections, which have the potential to rapidly reach new locations. An estimated 75% of infectious diseases originate in animals, including SARS, MERS, dengue, Ebola, Zika, influenza, and COVID-19.
Changing land-use patterns are increasing frequent interactions between people and once-remote animals. The infectious diseases segment in the LNP market is growing at a rapid rate due to the demand for new medicine delivery mechanisms. LNPs show promise in preventing mRNA from being broken down in the body, increasing treatment efficiency. In addition, lipid nanoparticles are also being tapped for developing treatments for infectious diseases like HIV and influenza. This has further contributed to the growth in the lipid nanoparticle raw materials market.
A rapidly aging population and rising risk factors associated with socioeconomic development, obesity, alcohol, tobacco consumption, and air pollution are causing a rise in global incidences of cancer. The WHO predicts that over 35 million people will be affected by cancer by 2050. The rise in cancer and lung disease cases is also fueling medical research and the development of new drugs and treatment protocols. Innovation in LNPs, especially in the gene editing space for lung disease and cystic fibrosis, is driving attention and funding.
Developments in siRNA encapsulated lipid nanoparticle (LNP) in vivo delivery systems are proving to be highly efficient at targeting respiratory syncytial virus (RSV) lung infections in vivo. LNPs offer promising new drug-delivery platforms by being able to encapsulate a variety of medications. All these factors are driving demand for raw materials used in the production of lipid nanoparticles.
Customize this study as per your requirement @ https://www.towardshealthcare.com/customization/5168
Increasing Adoption of LNPs in mRNA and Gene Editing Technology
LNPs are gaining popularity in delivering therapeutics, including those based on nucleic acids and mRNA. Advancements in mRNA technologies have changed the way new pharmaceuticals are being developed on a global scale. mRNA technology was widely used in the creation of COVID-19 vaccinations and shows promise in the development of more effective antigen therapies.
The development of new, lipid nanoparticle-based CRISPR-Cas9 carrier systems is also a game-changer in gene editing technology. Certain LNPs like Polyethylene glycol-phospholipid-modified cationic lipid nanoparticles show significant promise for efficient CRISPR-Cas9 delivery as a gene editing instrument.
Burst Release System Restrains the Lipid Nanoparticle Raw Materials Market Growth
While research and development in LNPs is showing promising results for more sophisticated drug delivery mechanisms, a major problem encountered with lipid nanoparticles is the burst release observed with these systems. Production-related factors such as temperature variations, changes in the lipid matrix, and surfactant makeup can change the release patterns of medication housed in the lipid nanoparticles.
Interactions with the reticuloendothelial system (RES) are also a major hurdle for the administration of LNPs. The administered nanoparticle prompts a biological response from the body, resulting in the formation of protein coronas. Certain elements (called opsonins) in the corona can vary the uptake of the coated materials by cells of the RES. The “molecular signature” left behind by opsonins in the corona can affect the routes of particle internalization. The routes taken can affect the medication’s volume of distribution, organ disposition, and rate of clearance from the bloodstream.
Complex Processes in Lipid Nanoparticle Productio
The production of lipid nanoparticles is a major challenge in the growth of the raw material market, lowering overall demand. Lipid dispersions have limited transdermal and hydrophilic drug delivery capacity. LNPs are also subject to polymorphic change, lowering the effectiveness of molecule delivery. The toxicity of lipid nanoparticles in certain cell types (e.g., retinal cells) has not yet been researched enough, raising questions about their use in the delivery mechanisms of certain medications. The high costs of lipid nanoparticle production also hinder the widespread adoption of the technology just yet.
Gene Therapy Creates Opportunity for the Lipid Nanoparticle Raw Materials Market
The application of LNPs in delivering gene editing therapies like CRISPR-Cas 9 shows promise in the treatment of genetic disorders and conditions like cystic fibrosis, sickle cell anemia, and muscular dystrophy. The use of LNPs is also being researched for deliveries of RNA-based therapies to treat neurological conditions such as Parkinson’s, amyotrophic lateral sclerosis (ALS), and Alzheimer’s. LNPs are proving instrumental in developing treatments for rare diseases where tailored therapies are necessary.
Lipid nanoparticles have gained traction in gene therapy as non-viral delivery systems demonstrating efficiency in delivering small-interfering RNAs (siRNAs) and DNA including cationic lipids, cell-penetrating peptides, liposomes, and cationic polymers. Cationic SLNs show promise for use in gene delivery due to the electrostatic interactions between the positive charges of the lipid and the negative charges of the DNA. These interactions cause the formation of lipoplexes which protects the DNA and directs it smoothly towards target cells.
Increased R&D and Government Funding in Lipid Nanoparticle Research
The promise shown by LNPs in both the pharmaceutical and biotechnology industries has led to increased spending on R&D. Increasing government funding is playing a crucial towards innovation in the space. Government funding initiatives by the National Institutes of Health (NIH) in North America have crossed over USD 228.4 million in the past three years. The U.S. Biomedical Advanced Research and Development Authority (BARDA) has also directed USD 150 million in funding for LNPs in 2022. Massachusetts-based Hopewell Therapeutics has also acquired $25 million in seed funding for developing LNPs for delivering nucleic acid cargos to treat lung diseases.
In Europe, AlgiPharma has received more than USD 46 million for research and development in LNPs in the form of grants from the EU’s 7th framework and Eurostars programs. The long-term promise showed by LNPs as effective drug delivery platforms is contributing to significant interest in the technology and attracting.
North America Leads the Lipid Nanoparticle Raw Materials Market
North America dominates in the global lipid nanoparticle raw materials market with a notable share. Several factors including significant research and development spending from the universities, biotechnology firms, and the government have helped the region dominate the market. Investment from the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services has also spurred advancements in lipid nanoparticle research. The global impact of Pfizer-BioNTech and Moderna's LNP-based mRNA COVID-19 vaccines have helped boost the industry significantly and generated much interest in lipid nanoparticle research.
- Currently, over 60% of the world’s active LNP clinical trials are taking place in North America with a 15% growth in LNP-related patents being filed in the region. North America is also home to several LNP-specialized contract development and manufacturing organizations that provide the required equipment and raw materials for research and development in the field.
Canada has also made a significant contribution to the expansion of the LNP raw material market. For instance, in April 2023, the government of Canada approved C$200 million in funding through development agencies for integrating AI in the development of agriculture and healthcare-based LNPs.
Asia-Pacific is the Fastest-Growing Region in the Lipid Nanoparticles Raw Materials Market
The Asia-Pacific houses the fastest-growing sector of the lipid nanoparticle raw materials market due to the growing pharmaceutical industry in India and China and increased government healthcare spending. A rise in incidences of infectious diseases and chronic conditions in the region has led to increased government budget allocations towards research and development of LNPs. In Asia, the number of cancer cases has gone up to 9.4 million, having doubled by 2019 in comparison to 1990. As more people get affected by such conditions, the demand for treatments, including those centered around lipid nanoparticles has gone up.
Government initiatives in South Korea in the past few years have focused on new drug delivery methods, including lipid nanoparticles, fostering a strong collaboration between the industry and academic research institutes. For instance, researchers in the Center for Nano Manufacturing and the Department of Nanoscience and Engineering at Inje University have focused on developing a solid lipid nanoparticle (SLN) system to enhance Curcumin bioavailability to utilize its anticancer properties.
By Product, the Kits Segment holds the Largest Share in Market
The kits segment was the largest in the market in 2023. Lipid nanoparticle kits can be used to create components for therapeutic uses that target specific cells and tissue types. Kits provide a way of continuously refining the manufacturing process to increase stability and payload delivery in lipid nanoparticle products. LNP kits are versatile with uses in cellular imaging, drug screening, and the creation of fluorescent probes and dyes, leading to a rise in their demand for academic and biotechnology research. The growth of the personalized medicine sector is contributing to the growth in the kit segment of the LNP market with the promise of increasingly customizable formulations.
The reagent segment is expected to be the fastest growing in the forecast period. Reagents enable researchers to establish a clinically relevant and scalable method for ex vivo gene delivery and editing as well as enable target validation. Reagents help in efficient knockout, and expression, and enhance cell viability, making them essential components of lipid nanoparticle research. Increasing investment in biotechnology-based R&D activities and the growing need for screening chronic diseases are contributing to the growth of this segment.
Browse More Insights of Towards Healthcare:
1. Consumer Genomics Market Size, Share and Trends Report
The global consumer genomics market size was estimated at US$ 1.57 billion in 2023 and is projected to grow to US$ 14.45 billion by 2033, rising at a compound annual growth rate (CAGR) of 24.85% from 2024 to 2033.
2. 503B Compounding Pharmacies Market Manufacturers Analysis Report
The global 503B compounding pharmacies market size was estimated at US$ 1.08 billion in 2023 and is projected to grow US$ 2.25 billion by 2033, rising at a compound annual growth rate (CAGR) of 7.63% from 2024 to 2033.
3. Computational Biology Market Size, Growth and Trends Report
The global computational biology market size was estimated at US$ 5.60 billion in 2023 and is projected to grow US$ 19.35 billion by 2033, rising at a compound annual growth rate (CAGR) of 13.20% from 2024 to 2033.
4. The global biomaterials market size was estimated at US$ 178.16 billion in 2023 and is projected to grow US$ 761.23 billion by 2033, rising at a compound annual growth rate (CAGR) of 15.63% from 2024 to 2033.
5. The global circulating tumor cells market size was estimated at US$ 11.49 billion in 2023 and is projected to grow US$ 41.27 billion by 2033, rising at a compound annual growth rate (CAGR) of 13.64% from 2024 to 2033.
6. The global gene synthesis market size was estimated at US$ 2.1 billion in 2023 and is projected to grow US$ 9.38 billion by 2033, rising at a compound annual growth rate (CAGR) of 16.14% from 2024 to 2033.
7. The global sequencing market size was estimated at US$ 15.59 billion in 2023 and is projected to grow US$ 115.85 billion by 2033, rising at a compound annual growth rate (CAGR) of 22.21% from 2024 to 2033.
8. The global retinal biologics market size was valued at US$ 23.18 billion in 2023 is expected to reach US$ 49.67 billion by 2033, at a compound annual growth rate (CAGR) of 7.92% from 2024 to 2033.
9. The cancer vaccines market was valued at US$ 10.21 billion in 2023 and is predicted to reach US$ 30.16 billion by the end of 2033, representing an impressive CAGR of 11.44% from 2024 to 2033.
10. The respiratory drugs market size is estimated to grow from 16.57 billion in 2023 to reach around USD 28.11 billion by 2032, registering a CAGR of 5.8% between 2024 and 2032.
Major Breakthroughs in Lipid Nanoparticle Raw Materials Market
- In May 2024, researchers from the University of Florida successfully conducted human clinical trials on four patients with a vaccine designed to reprogram the immune system to attack glioblastomas, a type of aggressive brain cancer. The preliminary vaccine uses mRNA and lipid nanoparticles to create personalized vaccines using a patient’s tumor and includes a new delivery mechanism for the same.
- In February 2024, researchers at the University of Pennsylvania created a specialized high-throughput screening platform for the assessment of mRNA encapsulated within lipid nanoparticles. The platform aims to provide quick screening of lipid nanoparticle libraries designed specifically for delivery through neurological pathways.
- In July 2023, Cytiva, a prominent player in biotechnology research, unveiled the NanoAssemblr commercial formulation system, designed for the production of LNP-based medicines on a clinical and commercial scale. The formulation system is designed to facilitate simplified manufacturing of lipid nanoparticle components.
Executive Summary
- Market Overview
- Key Findings
- Market Trends
- Market Opportunities
- Market Forecast
- Definition and Scope
- Methodology
- Assumptions and Limitations
- Drivers
- Restraints
- Opportunities
- Challenges
- Regulatory Landscape
By Product
- Ionizable Lipids
- PEGylated Lipids
- Sterol Lipids
- Neutral Phospholipids
- Kits
- Reagents
- Other Raw Materials
- Cancer
- Infectious Diseases
- Blood Diseases
- Others
- Therapeutics
- Research
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Asia Pacific
- Japan
- China
- India
- Australia
- Thailand
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- Saudi Arabia
- UAE
- Kuwait
By Product vs. By Disease Indication
- Ionizable Lipids
- Cancer
- Infectious Diseases
- Blood Diseases
- Others
- PEGylated Lipids
- Cancer
- Infectious Diseases
- Blood Diseases
- Others
- Sterol Lipids
- Cancer
- Infectious Diseases
- Blood Diseases
- Others
- Neutral Phospholipids
- Cancer
- Infectious Diseases
- Blood Diseases
- Others
- Kits
- Cancer
- Infectious Diseases
- Blood Diseases
- Others
- Reagents
- Cancer
- Infectious Diseases
- Blood Diseases
- Others
- Other Raw Materials
- Cancer
- Infectious Diseases
- Blood Diseases
- Others
- Ionizable Lipids
- Therapeutics
- Research
- PEGylated Lipids
- Therapeutics
- Research
- Sterol Lipids
- Therapeutics
- Research
- Neutral Phospholipids
- Therapeutics
- Research
- Kits
- Therapeutics
- Research
- Reagents
- Therapeutics
- Research
- Other Raw Materials
- Therapeutics
- Research
- Ionizable Lipids
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- PEGylated Lipids
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Sterol Lipids
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Neutral Phospholipids
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Kits
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Reagents
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Other Raw Materials
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Cancer
- Therapeutics
- Research
- Infectious Diseases
- Therapeutics
- Research
- Blood Diseases
- Therapeutics
- Research
- Others
- Therapeutics
- Research
- Cancer
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Infectious Diseases
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Blood Diseases
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Others
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Therapeutics
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
- Research
- North America: U.S., Canada
- Europe: U.K., Germany, France, Italy, Spain, Denmark, Sweden, Norway
- Asia Pacific: Japan, China, India, Australia, Thailand, South Korea
- Latin America: Brazil, Mexico, Argentina
- Middle East and Africa (MEA): South Africa, Saudi Arabia, UAE, Kuwait
Executive Summary
- Overview of Go-to-Market Strategies
- Key strategies for market entry and success
- Main tactics for achieving growth
- Key Strategic Goals
- Market share expansion
- Product differentiation and innovation
- Strengthening brand presence
- Summary of Strategic Approaches
- Overview of segmentation, differentiation, partnerships, and pricing strategies
- Segment Identification
- Key segments: therapeutics, research, disease indications
- Focus areas: emerging applications, high-growth markets
- Customer Profiling
- Profiles: pharmaceutical companies, biotech firms, research institutions
- Insights into customer needs and behavior
- Customized Solutions
- Tailored solutions for different segments
- Custom products and services based on specific needs
- Innovative Products
- Development of novel lipid formulations
- Unique product attributes and benefits
- Quality Assurance
- Rigorous quality control procedures
- Emphasis on product reliability and standards
- Collaborations
- Partnerships for product co-development
- Research institution collaborations
- Supply Chain Partnerships
- Alliances with suppliers and distributors
- Efficient supply chain management
- Regulatory Navigation
- Expertise in regulatory requirements (e.g., FDA, EMA)
- Guidance on compliance and approvals
- Compliance
- Adherence to regulations and quality standards
- Communication of compliance status
- Brand Positioning
- Positioning as a market leader
- Focus on innovation, quality, and service
- Educational Content
- Creation of white papers, case studies, and webinars
- Raising awareness and establishing thought leadership
- Direct Sales
- Building and managing a direct sales team
- Personalized service and customer engagement
- Distributor Networks
- Development of distributor relationships
- Expansion into new geographic regions
- Competitive Analysis
- Monitoring competitors’ strategies and market positions
- Identifying opportunities for differentiation
- Customer Feedback
- Collecting and analyzing feedback
- Using insights to enhance products and services
- R&D Investment
- Investment in research and development
- Exploration of new technologies and advancements
- Technology Adoption
- Use of advanced technologies and digital tools
- Implementation of new manufacturing techniques
- Regional Strategies
- Development of region-specific strategies
- Adaptation to local market conditions
- Local Presence
- Establishing local offices or partnerships
- Building relationships with local stakeholders
- Introduction to AI in the Market
- Overview of AI applications in lipid nanoparticle raw materials
- Benefits of AI integration
- AI in Research and Development
- Accelerating R&D processes with AI
- AI-driven innovation in lipid formulations
- Predictive analytics for product performance
- AI in Manufacturing Processes
- Automation and optimization of manufacturing
- Quality control through AI
- Enhancing production efficiency with AI
- AI in Market Analysis and Forecasting
- AI for market trend analysis
- Forecasting demand and identifying growth opportunities
- Competitive intelligence through AI
- AI in Customer Engagement and Personalization
- AI-driven customer insights and profiling
- Personalization of marketing strategies
- Enhancing customer experience with AI
- AI in Supply Chain Management
- AI for optimizing supply chain operations
- Predictive maintenance and logistics management
- AI for inventory management and demand forecasting
- Case Studies and Examples
- Successful AI integration in the lipid nanoparticle market
- Lessons learned and best practices
- Future Trends and Developments
- Emerging AI technologies in the market
- Future opportunities and challenges
- Conclusion
- Summary of AI’s impact on the market
- Strategic recommendations for AI adoption
- Market Share Analysis
- Key Strategies
- Company Profiles
- Polysciences, Inc.
- NOF AMERICA CORPORATION
- Biopharma PEG Scientific Inc.
- Creative Biolabs
- CordenPharma International
- Tebubio
- Avanti Polar Lipids
- Hopewell Therapeutics
- Echelon Biosciences
- Merck KGaA
- BroadPharm
- Cytiva
- Future Market Trends
- Forecast by Region
- Forecast by Product
- Forecast by Application
- List of Abbreviations
- Methodology
- References
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Browse our Brand-New Journals:
https://www.towardspackaging.com
https://www.towardsautomotive.com
https://www.precedenceresearch.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare
Get Our Freshly Printed Chronicle: https://www.healthcarewebwire.com