Koligo Therapeutics, Inc. announced that it has made KYSLECEL (autologous pancreatic islets) with a 24-hour expiration time available throughout the United States
|
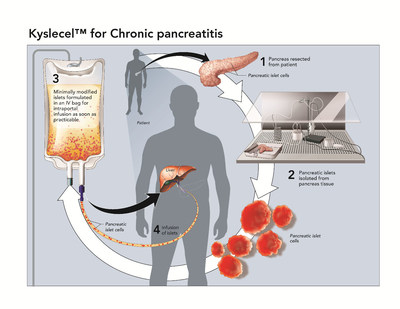
LOUISVILLE, Ky., July 16, 2019 /PRNewswire/ -- Koligo Therapeutics, Inc. announced that it has made KYSLECEL (autologous pancreatic islets) with a 24-hour expiration time available throughout the United States. KYSLECEL's 24-hour expiration time allows eligible patients to undergo TP-IAT at a qualified surgical center closer to home. Previously, TP-IAT had been limited to a handful of hospitals with specialized laboratories. TP-IAT can significantly reduce pain and the need for opioids, maintain glycemic control, and improve quality of life for patients with chronic or acute recurrent pancreatitis. KYSLECEL is immediately available at centers in New York City, Kentucky, and Indiana. Koligo is partnering with pancreas care centers across the U.S. and will announce availability as new referral centers open. Matthew Lehman, Koligo CEO: "The launch of KYSLECEL with a 24-hour shelf life reflects a significant advance in the ability to treat chronic and acute recurrent pancreatitis in the United States. We are excited to partner with leading pancreas care centers to make TP-IAT widely available to eligible patients." What is KYSLECEL?
KYSLECEL is a minimally-manipulated autologous cell-based product available in the United States and regulated by the FDA under section 361 of the PHS Act. KYSLECEL is produced according to current good tissue practices (cGTP) and in compliance with federal and state regulations. KYSLECEL is not routinely tested for transmissible infectious diseases. TP-IAT is a major surgery that carries significant risks. Review KYSLECEL Instructions for Use for important additional information. About Koligo Contact
SOURCE Koligo Therapeutics, Inc. |