Job Trends
Labor Market Reports
BioSpace’s 2026 U.S. Life Sciences Employment Outlook examines the state of the biopharma workforce amid ongoing funding pressure, elevated layoffs and cautious hiring sentiment, while highlighting early signals of stabilization and cautious optimism for the year ahead.
BioSpace’s 2025 Q4 U.S. Life Sciences Job Market update highlights early signs of stabilization in biopharma hiring, with modest gains in job postings, slowing layoffs, and cautiously improving sentiment heading into 2026.
BioSpace’s Q3 2025 U.S. Life Sciences Job Market Report reveals a turbulent quarter for biopharma hiring, with record declines in job postings, rising layoffs, and cautious employer sentiment shaping the industry’s employment landscape.
Now Hiring
Looking for a manufacturing job? Check out the BioSpace list of 11 companies hiring life sciences professionals like you.
If you’re looking for a biopharma job in Philadelphia, here are eight companies on BioSpace hiring right now.
Looking for a new opportunity in New Jersey? These nine companies have open roles that could be a great fit for you.
Career Advice
If workloads aren’t adjusted as needed, the company’s priorities are already compromised. Executive coach Angela Justice explores what happens when goals move forward without removing unnecessary work and what to do about it.
THE LATEST
Competition for biotech and pharma employment picked up year over year and remains strong, according to BioSpace data. A survey late last year showed that 64% of employed/contract and 96% of unemployed respondents will actively look for work in 2026.
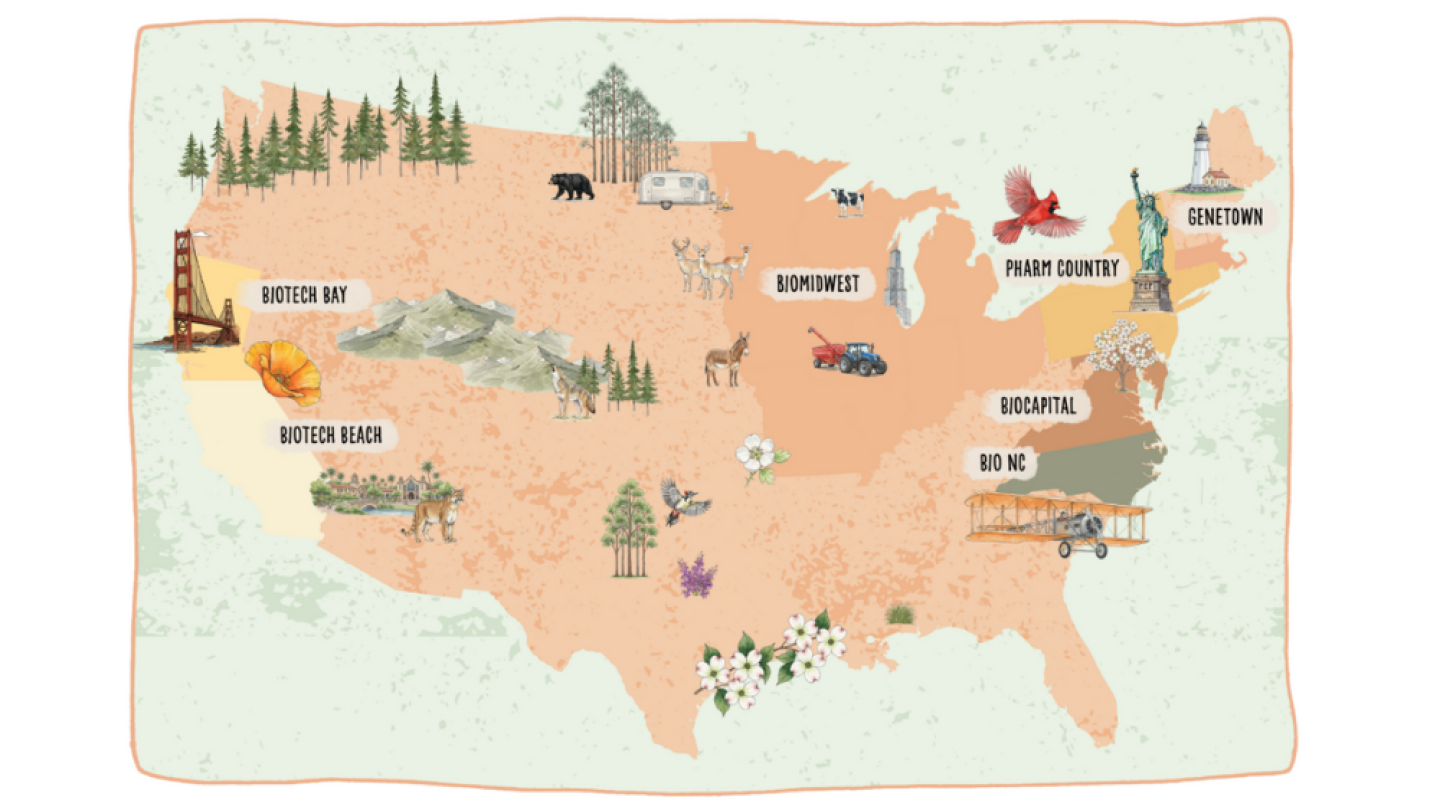
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
In this bonus episode, BioSpace’s Vice President of Marketing Chantal Dresner and Careers Editor Angela Gabriel take a look at Q4 job market performance and what it signals for 2026.
Less than six months after cutting 20% of its employees, Vedanta Biosciences has again laid off staff. According to one affected staffer, half of the Cambridge, Massachusetts–based biotech’s workforce is being cut while most of the rest are furloughed.
The past year saw manufacturing challenges pushed into the spotlight. BioSpace spoke to two executives who shared key issues facing those working in this area, from finding the right providers to dealing with regulatory uncertainty.
Johnson & Johnson’s second facility in Wilson, North Carolina, is part of a $55 billion push to make all advanced medicines used in the U.S. domestically.
While there had been hope layoffs would slow down in 2025, they continued at a fast pace, affecting even more people than in 2024, based on BioSpace tallies.
In 2025, made or projected biopharma workforce cuts affected about 42,700 employees, according to BioSpace tallies. BioSpace takes a deep dive into which companies and locations were impacted and speaks to experts about what to expect ahead—and why.
For the second month in a row, job postings on BioSpace increased in key biopharma disciplines. However, application rates also increased.
Job listings in the area have ticked up in the last month. These seven companies are hiring in South San Francisco right now, including scientist and clinical roles.