| |
NEW YORK, April 21, 2020 /PRNewswire/ -- Hoth Therapeutics Inc. (NASDAQ: HOTH), a biopharmaceutical company, today announced top line data from its Cutaneous Lupus Erythematosus (CLE) study, a chronic autoimmune skin disease, in partnership with Zylö Therapeutics, Inc. for topical administration of Anandamide (AEA)-loaded Z-pods™.
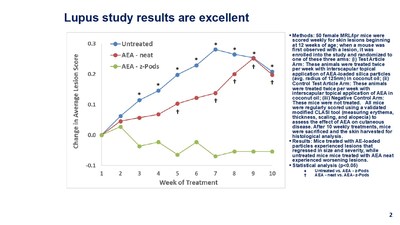
During the study, 50 female MRL/lpr mice that develop an autoimmune disease resembling systemic lupus erythematosus, a disease affecting over approximately 1.5 million Americans, were scored weekly for skin lesions beginning at 12 weeks of age. The mice were tested weekly for 10 weeks. The results show that mice treated with AEA-loaded particles experienced lesions that regressed in size and severity, while untreated mice treated with AEA-neat experienced worsening lesions.
When a mouse was first observed with a lesion, it was enrolled into the study and randomized to one of these three arms: (i) Test Article Arm: These animals were treated twice per week with interscapular topical application of AEA-loaded silica particles (avg. radius of 125nm) in coconut oil; (ii) Control Test Article Arm: These animals were treated twice per week with interscapular topical application of AEA in coconut oil; (iii) Negative Control Arm: These mice were not treated. All mice were regularly scored using a validated modified CLASI tool, measuring erythema, thickness, scaling, and alopecia, to assess the effect of AEA on cutaneous disease.
Mr. Robb Knie, Chief Executive Officer of Hoth, commented, "We are pleased to have concluded our preclinical study of CLE. Clearly, administering anandamide through the AEA-loaded Z-pods™ delivery system has significant lesion improvement in CLE patients. Together with the Zylö team, we will continue to move this treatment forward through to the clinic."
Hoth owns an exclusive license to develop Anandamide-loaded Z-pods™ for the treatment of CLE in North America. Anandamide, generally referred to as AEA, is one of the cannabinoids that the human body makes naturally. The Company also possesses rights related to geographic expansion and to follow-on indications such as psoriasis and rheumatoid arthritis.
About Hoth Therapeutics, Inc.
Hoth Therapeutics, Inc. is a clinical-stage biopharmaceutical company focused on developing new generation therapies for dermatological disorders. HOTH's pipeline has the potential to improve the quality of life for patients suffering from indications including atopic dermatitis, chronic wounds, psoriasis, asthma and acne. Hoth has recently entered into a Joint Development Agreement to further the development of vaccine prospects to prevent, intercept or treat the Coronavirus (COVID-19) based upon VaxCelerate, a self-assembling vaccine (SAV) platform exclusively licensed by Voltron from the Vaccine and Immunotherapy Center (VIC) at Massachusetts General Hospital (MGH). To learn more, please visit www.hoththerapeutics.com.
Forward Looking Statements
This press release includes "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements in this press release include, but are not limited to, statements that relate to the advancement and development of AEA-loaded Z-pods™, the commencement of clinical trials, the availability of data from clinical trials and other information that is not historical information. When used herein, words such as "anticipate", "being", "will", "plan", "may", "continue", and similar expressions are intended to identify forward-looking statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking. All forward-looking statements are based upon Hoth's current expectations and various assumptions. Hoth believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. Hoth may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various important factors, including, without limitation, market conditions and the factors described under the caption "Risk Factors" in Hoth's Form 10K for the period ending December 31, 2018, and Hoth's other filings made with the Securities and Exchange Commission. Consequently, forward-looking statements should be regarded solely as Hoth's current plans, estimates and beliefs. Investors should not place undue reliance on forward-looking statements. Hoth cannot guarantee future results, events, levels of activity, performance or achievements. Hoth does not undertake and specifically declines any obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by law.
Investor Contact:
Phone: (646) 756-2997
Email: investorrelations@hoththerapeutics.com
www.hoththerapeutics.com
KCSA Strategic Communications
Valter Pinto, Managing Director
(212) 896-1254
Hoth@kcsa.com
 View original content to download multimedia:http://www.prnewswire.com/news-releases/hoth-therapeutics-announces-top-line-preclinical-data-from-cutaneous-lupus-erythematosus-cle-study-301044299.html View original content to download multimedia:http://www.prnewswire.com/news-releases/hoth-therapeutics-announces-top-line-preclinical-data-from-cutaneous-lupus-erythematosus-cle-study-301044299.html
SOURCE Hoth Therapeutics, Inc.
|
|






