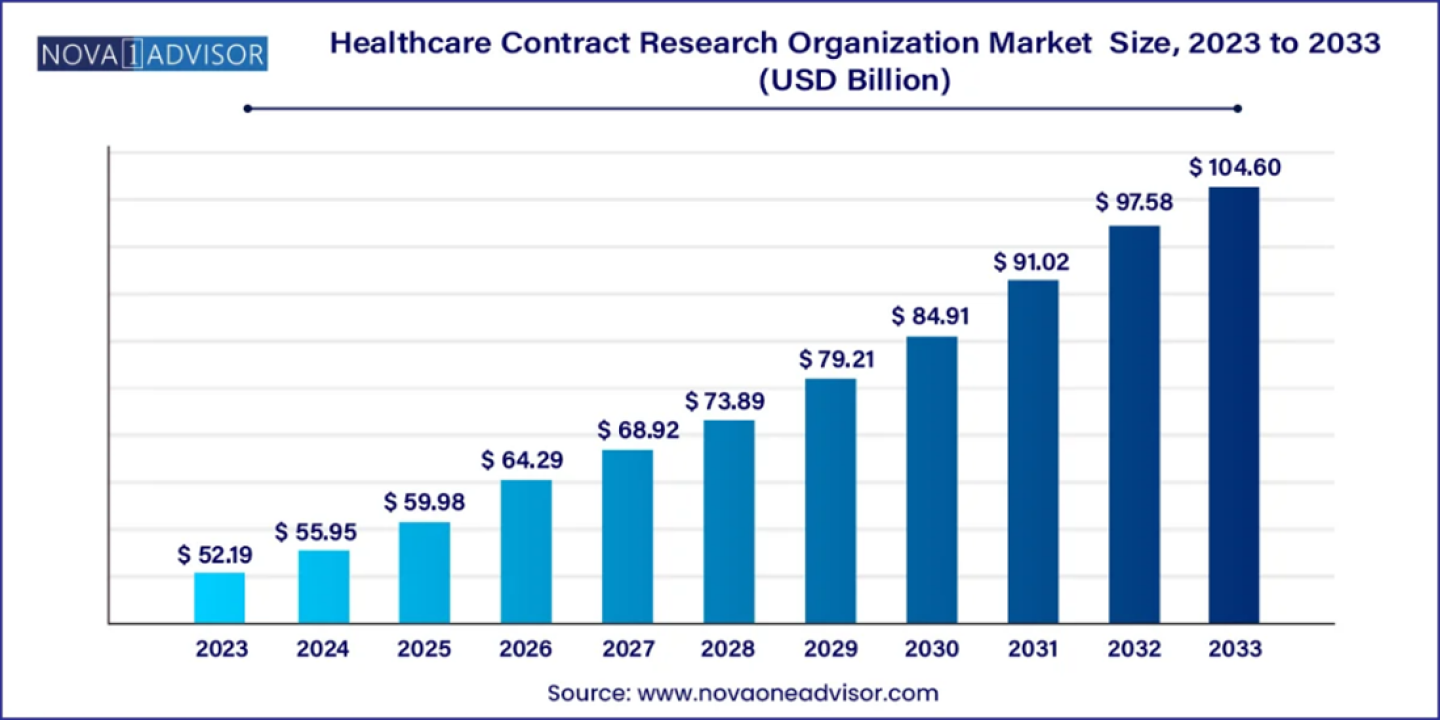
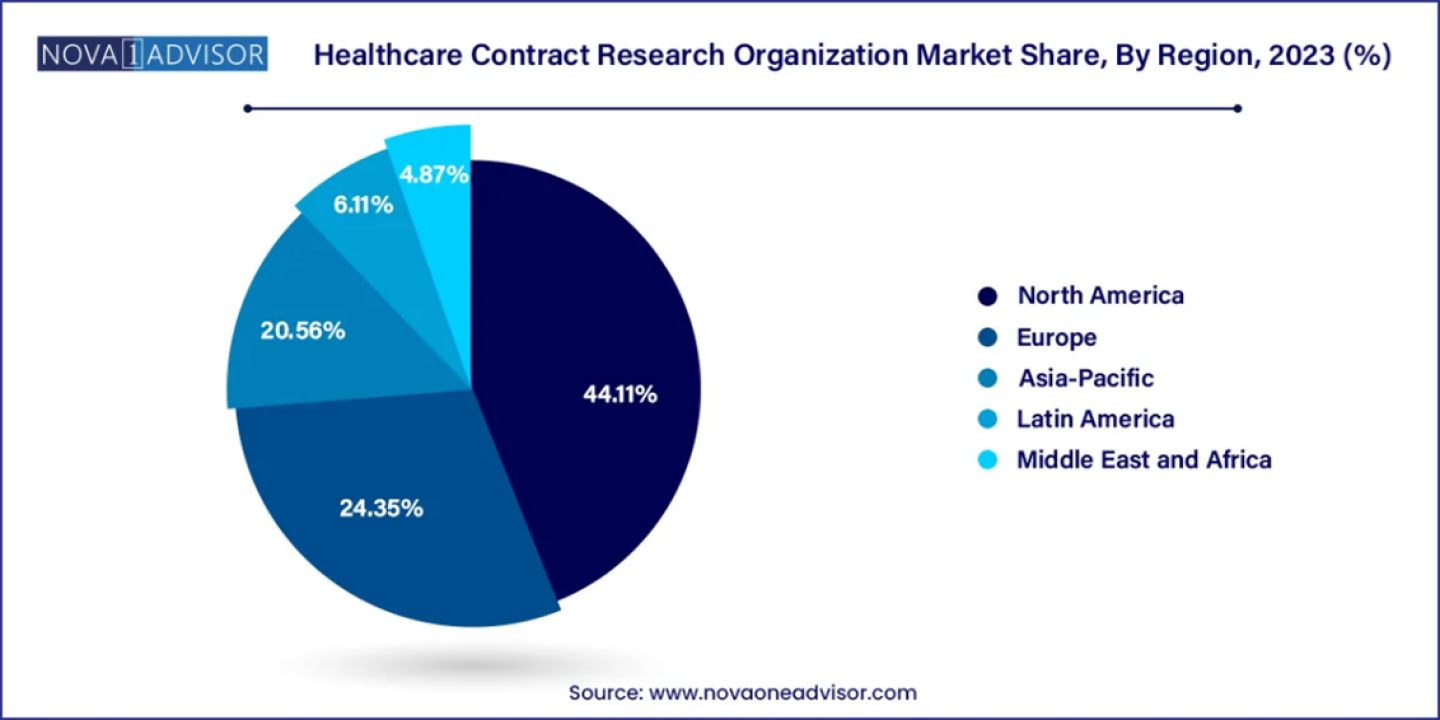
According to latest report, the global healthcare contract research organization (CRO) market size was USD 52.19 billion in 2023, calculated at USD 55.95 billion in 2024 and is expected to reach around USD 104.60 billion by 2033, expanding at a CAGR of 7.2% from 2024 to 2033. North America dominated the healthcare contract research organization market with the largest revenue share of 38% in 2023.
Contract Research Organizations (CROs) are integral to the drug development process, offering a full range of services that span every phase of clinical trials. They play a crucial role in developing robust study protocols, ensuring scientific rigor from trial inception. CROs employ effective strategies for patient identification and enrollment, critical for meeting trial objectives.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/6458
Market Overview
The healthcare contract research organization market is experiencing rapid growth driven by increasing demand from pharmaceutical, biotechnology, and medical device industries. CROs specialize in providing a comprehensive array of services essential for clinical trials, including regulatory affairs, clinical trial planning, site selection and initiation, recruitment support, clinical monitoring, data management, trial logistics, biostatistics, medical writing, and project management. These services are crucial for managing and executing clinical research studies efficiently, supporting product development from inception to market approval. CROs play a pivotal role in navigating complex regulatory landscapes and ensuring adherence to ethical standards, safeguarding the rights and well-being of study participants. Their expertise and ability to streamline processes contribute significantly to the market's growth by enabling efficient and compliant clinical trial operations across global healthcare sectors.
· In February 2024, European CRO FGK expanded into the UK through the acquisition of Clinicology.
· In July 2023, Catalyst Clinical Research announced the acquisition of Genpro Research.
· In August 2023, Kohlberg signed a definitive agreement to acquire a majority stake in Worldwide Clinical Trials.
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/6458
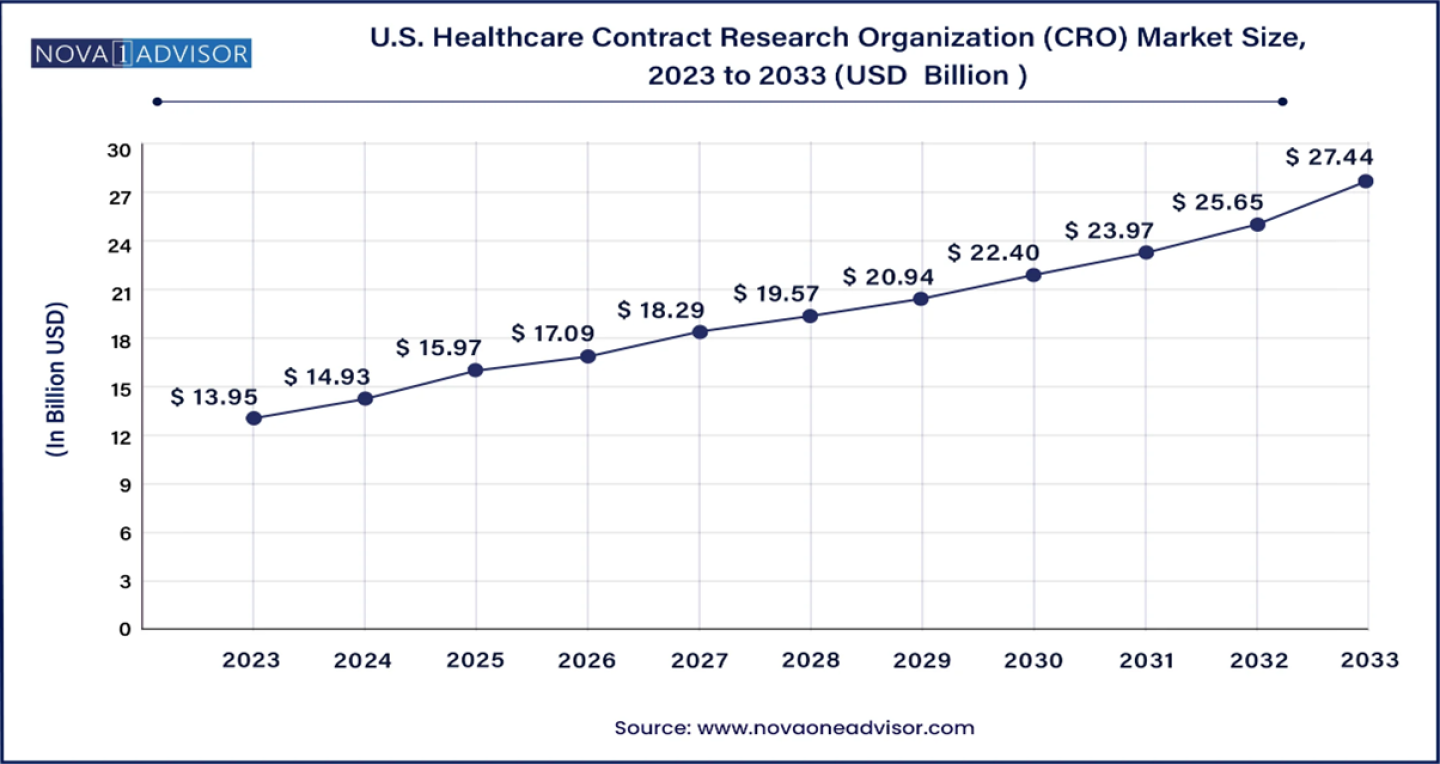
U.S. Healthcare Contract Research Organization (CRO) Market Size to Hit USD 27.44 Bn by 2033
The U.S. healthcare contract research organization (CRO) market size was estimated at USD 13.95 billion in 2023 and is projected to hit around USD 27.44 billion by 2033, growing at a CAGR of 7.0% during the forecast period from 2024 to 2033.
North America stands as a leader in the healthcare contract research organization market, fueled by robust contributions from prominent companies such as IQVIA, known for its extensive service portfolio in clinical research advancement. Companies like ClaraHealth, with their patient-centric recruitment acceleration platform, play a pivotal role in enhancing CRO operations across the US and globally. In Canada, the pharmaceutical industry's focus on innovation is pivotal, aiming to broaden access to cutting-edge medicines, vaccines, and treatments nationwide. This collaborative effort is not only driving advancements in healthcare but also positively impacting communities throughout the region, reinforcing North America's leadership in the global healthcare contract research organization market.
Asia Pacific emerges as the second most dominant region in the healthcare contract research organization market, driven by a robust network of specialized service providers facilitating comprehensive drug discovery and development programs for pharmaceutical and biotechnology companies. These include Discovery CROs, Pre-Clinical CROs, Clinical CROs, and those offering bioequivalence and bioavailability services. Outsourcing research and development activities to CROs allows companies to enhance operational efficiency and focus on core competencies. In China, the expansion of health insurance coverage to over 95% of the population reflects significant strides in healthcare accessibility, supported by decades of economic growth and rising personal incomes. This socio-economic progress has spurred increased demand for improved healthcare services, underscoring Asia Pacific's pivotal role in driving growth and innovation within the global CRO market.
Report Highlights
By Service
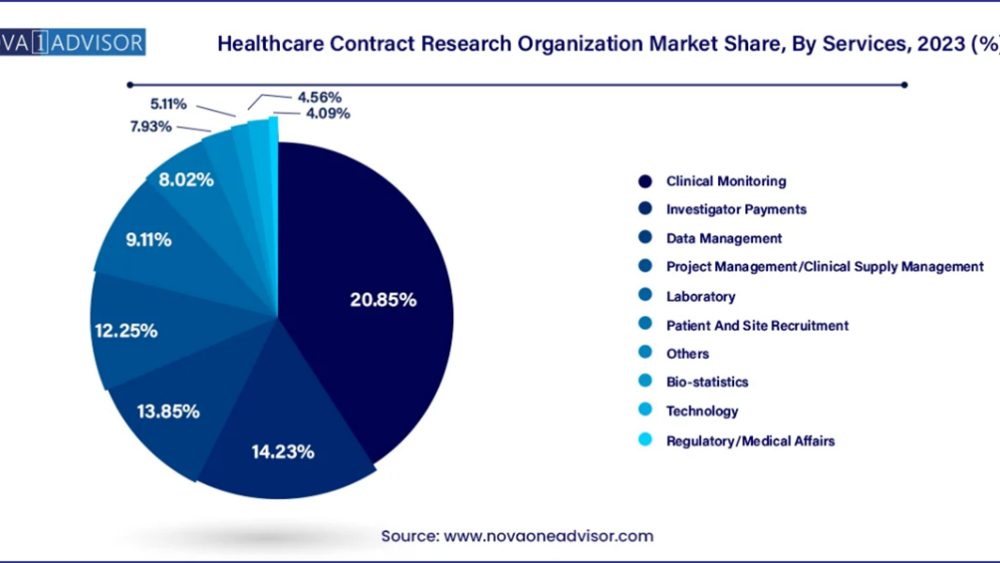
The clinical monitoring segment stands out as a dominant force in the global healthcare contract research organization market. Effective clinical monitoring is pivotal for ensuring the success of clinical trials and accelerating the introduction of new drugs to market. This service involves meticulous oversight and assessment of trial activities, tailored to meet the unique requirements of each study. By providing comprehensive monitoring services, CROs play a crucial role in maintaining trial integrity, adherence to protocols, and regulatory compliance, thereby facilitating efficient and successful drug development processes for pharmaceutical and biotechnology companies worldwide.
The regulatory/medical affairs segment is poised for growth with a projected CAGR in the forecast period within the healthcare contract research organization market. Regulatory affairs has evolved as a critical profession aimed at safeguarding public health by regulating the safety and efficacy of various products, including pharmaceuticals, medical devices, and cosmetics. Governments enforce stringent regulations to ensure products meet quality standards, while companies strive to comply to maintain public trust and health benefits. As regulatory complexities increase globally, CROs specializing in regulatory affairs play a pivotal role in navigating these frameworks, offering expertise in compliance, submission processes, and strategic advice. This segment's anticipated growth underscores its importance in facilitating regulatory compliance and supporting the successful development and market approval of healthcare products worldwide.
By Type
The clinical segment emerged as the dominant category in the global healthcare contract research organization market. CROs possess specialized knowledge, capabilities, and established processes essential for the successful development and execution of clinical trials, ensuring adherence to rigorous national and international standards. Partnering with a CRO equips pharmaceutical and biotechnology companies with innovative tools that enhance operational efficiencies, resulting in reduced trial timelines and costs. While clinical CROs focus on comprehensive trial management services, laboratory CROs specialize in drug discovery, manufacturing, and bioanalytical services, collectively bolstering the capabilities necessary for advancing medical research and therapeutic innovations on a global scale.
The pre-clinical segment is anticipated to experience the fastest growth during the forecast period within the healthcare contract research organization market. Pre-clinical research encompasses the crucial stages of development and testing that precede human trials, focusing on verifying the safety and efficacy of new products. This process involves meticulous protocol creation and initial research phases to assess product safety through animal studies. Pre-clinical studies play a pivotal role in evaluating the potential of therapeutic drugs or strategies before progressing to clinical trials, ensuring that only the most promising candidates advance to human testing. As demand for early-stage research continues to expand, CROs specializing in pre-clinical services are poised to play a pivotal role in accelerating drug development timelines and enhancing research efficiencies, thereby driving growth in the global healthcare contract research organization market.
By application:
The oncology segment is observed to grow at a notable rate in the healthcare contract research organization market. The rise of precision medicine, which tailors treatments based on individual genetic profiles, particularly in oncology, requires extensive data collection, analysis, and clinical trial support from specialized CROs. The complexity and rigorous regulatory requirements for oncology drugs necessitate specialized expertise and resources that CROs can provide, including navigating clinical trial design, patient recruitment, and compliance with regulatory standards. Innovations in cancer treatment, such as immunotherapies, targeted therapies, and personalized medicine, require extensive research and development, leading to a greater need for specialized CRO services.
Immediate Delivery Available, Get Full Access@ https://www.novaoneadvisor.com/report/checkout/6458
Market Dynamics
Driver
Integral Support in Drug Development
Contract Research Organizations (CROs) contribute significantly to the healthcare sector by providing comprehensive services across drug development, laboratory operations, and lifecycle management. They offer essential pre-clinical drug development services such as pharmacology and toxicology testing, development of drug delivery devices, and conducting shipping tests. CROs specialize in data management, regulatory affairs, and quality assurance, ensuring compliance and efficiency throughout the drug development process. CROs play a crucial role in accelerating drug development timelines and reducing costs, particularly beneficial for small businesses. Their cost-efficient services compared to in-house development enable companies to expedite the evaluation of new drugs while mitigating expenses. This capability enhances the attractiveness of CROs, driving growth in the healthcare contract research organization market by supporting efficient and streamlined drug development processes.
Restraint
Complex Regulatory Compliance
Complex regulatory compliance poses a significant restraint on the growth of healthcare Contract Research Organizations (CROs). CROs play a crucial role in managing regulatory affairs for clinical trials, ensuring compliance with intricate regulations and guidelines governing the industry. Their expertise and knowledge are essential in navigating these complexities to ensure that trials meet stringent standards and expectations. Rigorous regulatory environment can slow down operational timelines and increase costs, presenting challenges for CROs aiming to streamline processes and expand their market presence. Addressing these regulatory hurdles through enhanced efficiency and compliance strategies is essential for sustained growth in the healthcare contract research organization market.
Opportunity
Technology Transformation
Investing in digital technologies presents a significant opportunity for Contract Research Organizations (CROs) to enhance collaboration across the clinical trial ecosystem and elevate their leadership position. Technology integration enables CROs to streamline traditionally manual administrative tasks, improving trial success by enhancing transparency, controlling spending, accelerating start-up timelines, and generating cost savings for sponsors. This technological evolution not only drives operational efficiency but also strengthens the value proposition of CROs, positioning them to capitalize on the growing demand for streamlined and tech-enabled clinical trial services.
Healthcare Contract Development And Manufacturing Organization Market Size (USD 630.87 Billion) Report by 2033
Oncology Market Size (USD 521.60 Billion) Report by 2033
Pharmaceutical CDMO Market Size (USD 295.95 Billion) Report by 2033
Biopharmaceutical Market Size (USD 1,101.77 Billion) Report by 2032
Biopharmaceutical CMO And CRO Market Size (USD 65.36 Billion ) Report by 2033
Active Pharmaceutical Ingredient CDMO Market Size (USD 249.22 Billion ) Report by 2033
Cell And Gene Therapy CDMO Market Size (USD 69.11 Billion ) Report by 2033
Advanced Therapy Medicinal Products CDMO Market Size (USD 34.53 Billion ) Report by 2033
Biotechnology Market Size (USD 5.68 Trillion ) Report by 2033
Clinical Trials Market Size (USD 153.59 Billion) Report by 2033
Immediate Delivery Available | Buy This Premium Research
https://www.novaoneadvisor.com/report/checkout/6458
Healthcare Contract Research Organization Market Top Key Companies:
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the Healthcare Contract Research Organization market.
By Type
About Us
Nova One Advisor is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Nova One Advisor has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defines, among different ventures present globally.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Email: sales@novaoneadvisor.com
Web: https://www.novaoneadvisor.com/
Contract Research Organizations (CROs) are integral to the drug development process, offering a full range of services that span every phase of clinical trials. They play a crucial role in developing robust study protocols, ensuring scientific rigor from trial inception. CROs employ effective strategies for patient identification and enrollment, critical for meeting trial objectives.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/6458
Market Overview
The healthcare contract research organization market is experiencing rapid growth driven by increasing demand from pharmaceutical, biotechnology, and medical device industries. CROs specialize in providing a comprehensive array of services essential for clinical trials, including regulatory affairs, clinical trial planning, site selection and initiation, recruitment support, clinical monitoring, data management, trial logistics, biostatistics, medical writing, and project management. These services are crucial for managing and executing clinical research studies efficiently, supporting product development from inception to market approval. CROs play a pivotal role in navigating complex regulatory landscapes and ensuring adherence to ethical standards, safeguarding the rights and well-being of study participants. Their expertise and ability to streamline processes contribute significantly to the market's growth by enabling efficient and compliant clinical trial operations across global healthcare sectors.
· In February 2024, European CRO FGK expanded into the UK through the acquisition of Clinicology.
· In July 2023, Catalyst Clinical Research announced the acquisition of Genpro Research.
· In August 2023, Kohlberg signed a definitive agreement to acquire a majority stake in Worldwide Clinical Trials.
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/6458
U.S. Healthcare Contract Research Organization (CRO) Market Size to Hit USD 27.44 Bn by 2033
The U.S. healthcare contract research organization (CRO) market size was estimated at USD 13.95 billion in 2023 and is projected to hit around USD 27.44 billion by 2033, growing at a CAGR of 7.0% during the forecast period from 2024 to 2033.
North America stands as a leader in the healthcare contract research organization market, fueled by robust contributions from prominent companies such as IQVIA, known for its extensive service portfolio in clinical research advancement. Companies like ClaraHealth, with their patient-centric recruitment acceleration platform, play a pivotal role in enhancing CRO operations across the US and globally. In Canada, the pharmaceutical industry's focus on innovation is pivotal, aiming to broaden access to cutting-edge medicines, vaccines, and treatments nationwide. This collaborative effort is not only driving advancements in healthcare but also positively impacting communities throughout the region, reinforcing North America's leadership in the global healthcare contract research organization market.
- In September 2023, Cerba HealthCare announced its acquisition of Canadian Contract Research Laboratory CIRION BioPharma Research, expanding bioanalytical capabilities for complex clinical trials.
Asia Pacific emerges as the second most dominant region in the healthcare contract research organization market, driven by a robust network of specialized service providers facilitating comprehensive drug discovery and development programs for pharmaceutical and biotechnology companies. These include Discovery CROs, Pre-Clinical CROs, Clinical CROs, and those offering bioequivalence and bioavailability services. Outsourcing research and development activities to CROs allows companies to enhance operational efficiency and focus on core competencies. In China, the expansion of health insurance coverage to over 95% of the population reflects significant strides in healthcare accessibility, supported by decades of economic growth and rising personal incomes. This socio-economic progress has spurred increased demand for improved healthcare services, underscoring Asia Pacific's pivotal role in driving growth and innovation within the global CRO market.
Report Highlights
By Service
The clinical monitoring segment stands out as a dominant force in the global healthcare contract research organization market. Effective clinical monitoring is pivotal for ensuring the success of clinical trials and accelerating the introduction of new drugs to market. This service involves meticulous oversight and assessment of trial activities, tailored to meet the unique requirements of each study. By providing comprehensive monitoring services, CROs play a crucial role in maintaining trial integrity, adherence to protocols, and regulatory compliance, thereby facilitating efficient and successful drug development processes for pharmaceutical and biotechnology companies worldwide.
The regulatory/medical affairs segment is poised for growth with a projected CAGR in the forecast period within the healthcare contract research organization market. Regulatory affairs has evolved as a critical profession aimed at safeguarding public health by regulating the safety and efficacy of various products, including pharmaceuticals, medical devices, and cosmetics. Governments enforce stringent regulations to ensure products meet quality standards, while companies strive to comply to maintain public trust and health benefits. As regulatory complexities increase globally, CROs specializing in regulatory affairs play a pivotal role in navigating these frameworks, offering expertise in compliance, submission processes, and strategic advice. This segment's anticipated growth underscores its importance in facilitating regulatory compliance and supporting the successful development and market approval of healthcare products worldwide.
By Type
The clinical segment emerged as the dominant category in the global healthcare contract research organization market. CROs possess specialized knowledge, capabilities, and established processes essential for the successful development and execution of clinical trials, ensuring adherence to rigorous national and international standards. Partnering with a CRO equips pharmaceutical and biotechnology companies with innovative tools that enhance operational efficiencies, resulting in reduced trial timelines and costs. While clinical CROs focus on comprehensive trial management services, laboratory CROs specialize in drug discovery, manufacturing, and bioanalytical services, collectively bolstering the capabilities necessary for advancing medical research and therapeutic innovations on a global scale.
The pre-clinical segment is anticipated to experience the fastest growth during the forecast period within the healthcare contract research organization market. Pre-clinical research encompasses the crucial stages of development and testing that precede human trials, focusing on verifying the safety and efficacy of new products. This process involves meticulous protocol creation and initial research phases to assess product safety through animal studies. Pre-clinical studies play a pivotal role in evaluating the potential of therapeutic drugs or strategies before progressing to clinical trials, ensuring that only the most promising candidates advance to human testing. As demand for early-stage research continues to expand, CROs specializing in pre-clinical services are poised to play a pivotal role in accelerating drug development timelines and enhancing research efficiencies, thereby driving growth in the global healthcare contract research organization market.
By application:
The oncology segment is observed to grow at a notable rate in the healthcare contract research organization market. The rise of precision medicine, which tailors treatments based on individual genetic profiles, particularly in oncology, requires extensive data collection, analysis, and clinical trial support from specialized CROs. The complexity and rigorous regulatory requirements for oncology drugs necessitate specialized expertise and resources that CROs can provide, including navigating clinical trial design, patient recruitment, and compliance with regulatory standards. Innovations in cancer treatment, such as immunotherapies, targeted therapies, and personalized medicine, require extensive research and development, leading to a greater need for specialized CRO services.
Immediate Delivery Available, Get Full Access@ https://www.novaoneadvisor.com/report/checkout/6458
Market Dynamics
Driver
Integral Support in Drug Development
Contract Research Organizations (CROs) contribute significantly to the healthcare sector by providing comprehensive services across drug development, laboratory operations, and lifecycle management. They offer essential pre-clinical drug development services such as pharmacology and toxicology testing, development of drug delivery devices, and conducting shipping tests. CROs specialize in data management, regulatory affairs, and quality assurance, ensuring compliance and efficiency throughout the drug development process. CROs play a crucial role in accelerating drug development timelines and reducing costs, particularly beneficial for small businesses. Their cost-efficient services compared to in-house development enable companies to expedite the evaluation of new drugs while mitigating expenses. This capability enhances the attractiveness of CROs, driving growth in the healthcare contract research organization market by supporting efficient and streamlined drug development processes.
Restraint
Complex Regulatory Compliance
Complex regulatory compliance poses a significant restraint on the growth of healthcare Contract Research Organizations (CROs). CROs play a crucial role in managing regulatory affairs for clinical trials, ensuring compliance with intricate regulations and guidelines governing the industry. Their expertise and knowledge are essential in navigating these complexities to ensure that trials meet stringent standards and expectations. Rigorous regulatory environment can slow down operational timelines and increase costs, presenting challenges for CROs aiming to streamline processes and expand their market presence. Addressing these regulatory hurdles through enhanced efficiency and compliance strategies is essential for sustained growth in the healthcare contract research organization market.
Opportunity
Technology Transformation
Investing in digital technologies presents a significant opportunity for Contract Research Organizations (CROs) to enhance collaboration across the clinical trial ecosystem and elevate their leadership position. Technology integration enables CROs to streamline traditionally manual administrative tasks, improving trial success by enhancing transparency, controlling spending, accelerating start-up timelines, and generating cost savings for sponsors. This technological evolution not only drives operational efficiency but also strengthens the value proposition of CROs, positioning them to capitalize on the growing demand for streamlined and tech-enabled clinical trial services.
- In February 2024, Avance Clinical and Ryght partnered to integrate novel GenAI technologies into clinical research networks.
Healthcare Contract Development And Manufacturing Organization Market Size (USD 630.87 Billion) Report by 2033
Oncology Market Size (USD 521.60 Billion) Report by 2033
Pharmaceutical CDMO Market Size (USD 295.95 Billion) Report by 2033
Biopharmaceutical Market Size (USD 1,101.77 Billion) Report by 2032
Biopharmaceutical CMO And CRO Market Size (USD 65.36 Billion ) Report by 2033
Active Pharmaceutical Ingredient CDMO Market Size (USD 249.22 Billion ) Report by 2033
Cell And Gene Therapy CDMO Market Size (USD 69.11 Billion ) Report by 2033
Advanced Therapy Medicinal Products CDMO Market Size (USD 34.53 Billion ) Report by 2033
Biotechnology Market Size (USD 5.68 Trillion ) Report by 2033
Clinical Trials Market Size (USD 153.59 Billion) Report by 2033
Immediate Delivery Available | Buy This Premium Research
https://www.novaoneadvisor.com/report/checkout/6458
Healthcare Contract Research Organization Market Top Key Companies:
- QVIA
- LabCorp
- Charles River Laboratories
- WuXiAppTec
- Syneos Health
- Parexel International
- PPD
- ICON Plc
- Medpace Holdings
- SGS
- PSI CRO AG
- Axcent Advanced Analytics
- BIO Agile Therapeutics
- Firma Clinical Research
- Acculab Life Sciences
- In May 2023, Charles River Laboratories International, Inc. and Wheeler Bio, Inc. agreed to introduce RightSourceSM at Wheeler Bio's manufacturing facility in Oklahoma City. RightSource, situated at a client location, represents a versatile biologics testing laboratory overseen and supervised by Charles River. This initiative aims to enhance the accessibility of swift, dependable quality control services to a more extensive spectrum of companies.
- In January 2023, Bruker Corporation and Switzerland-based biopharma CRO Biognosys AG announced a strategic partnership for biologics-based clinical research. This partnership helped Biognosys AG plan to open its first laboratory for advanced proteomics outsourcing services in the U.S.
- In October 2023, Curavit launched a Health Economics and Outcomes Research (HEOR) practice for pharmaceutical clinical trials, recognizing its growing importance in digital therapeutics.
- In March 2023, LEO Pharma and ICON entered into a strategic partnership to enhance clinical trial execution in medical dermatology.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the Healthcare Contract Research Organization market.
By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Patient And Site Recruitment
- Technology
- Others
- Oncology
- Cardiovascular
- Autoimmune/Inflammation
- Central nervous system (CNS)
- Dermatology
- Infectious diseases
- Diabetes
- Pain
- Others
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
- Others
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
About Us
Nova One Advisor is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Nova One Advisor has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defines, among different ventures present globally.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Email: sales@novaoneadvisor.com
Web: https://www.novaoneadvisor.com/