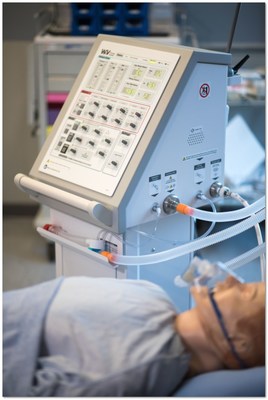
StarFish Medical announced that the Winnipeg Ventilator 2.0 has received a Health Canada COVID-19 Medical Device Authorization under the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19 made by the Minister of Health on March 18, 2020.
VICTORIA, BC, Sept. 25, 2020 /PRNewswire/ - StarFish Medical, Canada's largest medical device design, development, and manufacturing service provider, announced today that the Winnipeg Ventilator 2.0 has received a Health Canada COVID-19 Medical Device Authorization under the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19 made by the Minister of Health on March 18, 2020.
The approval allows Canadian Emergency Ventilators Inc. (CEV), as the manufacturer of record, to ship ventilator units to the Public Health Authority of Canada, starting immediately.
"Our goal with the Winnipeg Ventilator 2.0 is to deliver a fully featured ICU ventilator that could save patients' lives, be manufactured in Canada in the shortest time possible, and not disrupt the supply chain for existing ventilators", said StarFish Medical CEO and Founder, Scott Phillips. "To do that, we started with proven technology (original Winnipeg Ventilator designed by Dr. Magdy Younes), updated the design to incorporate technical advances and use non-medical supplier components, all while drawing upon a network of companies we have worked with for over 20 years. The pioneering work of Dr. Younes, and the support of Cerebra Health with clinical input and upcoming clinical trials is invaluable."
John Walmsley, StarFish Medical EVP Strategic Relationships, shares the importance of teamwork in the project's rapid progress, "Our supply chain moved quickly and diligently to discover what supplies and services were available, while our engineers worked with available components to create and build the design. We used 106 StarFish employees on the project and over 100 people at key vendors including Dometic, Advanced Test Automation, Yorkville, Dorigo Systems, Powersonic Industries and EM Dynamics. Having designed the product, we were very happy to have Celestica on board to coordinate with our supply chain and begin to bring on board their vendor network for manufacture."
Earl Gardiner, Executive Chairman & Founder of Cerebra Health points to team collaboration as a key success factor. "From the very beginning, Cerebra Health has been committed to supporting the StarFish vision of designing and engineering a high quality ICU ventilator, one that any respiratory specialist would readily use on a patient both during and after the pandemic has passed. They have done a masterful job, while working under extreme time and supply challenges. We are honoured to contribute to Canada's response to Covid."
StarFish presented the Ventilator design to expert review panels convened by NGen and ISED to very positive and encouraging feedback.
Dr Younes tested the updated version of his ventilator calling it a 'masterpiece indeed."
"StarFish Medical stepped up early on with their commitment to produce a made-in-Canada ventilator design that will help save the lives of COVID-19 patients battling the disease. Today's announcement is a testament to our Supercluster innovation program ensuring that industry has the flexibility and resources it needs. I am glad we were able to support StarFish Medical in producing this important lifesaving equipment." – The Honourable Navdeep Bains, Minister of Innovation, Science and Industry
"As the leader of Canada's Advanced Manufacturing Supercluster, NGen is committed to building world-leading advanced manufacturing capabilities in Canada", says Jayson Myers, NGen's Chief Executive Officer. "The Canadian Pandemic Ventilator project led by StarFish Medical is a shining example of how Canadian companies have responded rapidly to the COVID-19 crisis, combining their engineering, technology, and manufacturing capabilities to develop and produce an innovative solution that will help save lives. NGen has been a proud supporter of the Pandemic Ventilator project since we funded its launch three months ago. Health Canada's approval will now let the project partners move into manufacturing and get their ventilator into the hands of the patients who need them most."
"As soon as we received the StarFish innovative and manufacturable design for the new Winnipeg Ventilator, we leveraged our engineering, supply chain, and certified manufacturing expertise to source critical parts and begin production of 7,500 units without delay," said Kevin Walsh, Vice President, HealthTech, Celestica. "These ventilators are essential to treating critically-ill COVID-19 patients, so speeding time-to-market has never been more critical. We're proud to play a critical role in ensuring that StarFish meets its commitment to supply its ventilators to hospitals throughout Canada."
The Interim Order allows the Minister to permit the exceptional importation and sale of drugs, medical devices (including ventilators), and foods for special dietary purposes that do not fully comply with Canadian requirements, but are manufactured according to comparable standards.
StarFish Medical, on behalf of Canadian Emergency Ventilators, and Innovation, Science and Economic Development Canada plan to seek and receive full Health Canada approval to enable sale and use of the new ventilator beyond the Interim order authorization. To learn more about the ventilator project or license opportunities, please visit https://thewinnipegventilator.com/ or contact licensing@thewinnipegventilator.com.
About StarFish Medical
Empowering Medtech Innovation®
StarFish Medical is a full service Medical Device Design company offering design, development, and manufacturing services based in Toronto and Victoria. We use our Pathfinder™ process to reduce wasted effort and increase success for medical device product definition, technical engineering, and product development. Prototype and volume production are delivered within an ISO 13485 certified Quality Management System and an FDA registered manufacturing and clean room facility. www.starfishmedical.com
View original content to download multimedia:http://www.prnewswire.com/news-releases/health-canada-certifies-winnipeg-ventilator-2-0-for-immediate-distribution-to-support-the-needs-of-covid-19-patients-301138022.html
SOURCE StarFish Medical