SAN DIEGO, May 27, 2015 /PRNewswire/ -- Halozyme Therapeutics, Inc. (NASDAQ: HALO) and Ventana Medical Systems, Inc. (Ventana), a member of the Roche Group, announced today a global agreement to collaborate on the development of, and for Ventana to ultimately commercialize, a companion diagnostic assay for use with Halozyme's investigational new drug, PEGPH20.
The Ventana assay will be used to identify high levels of hyaluronan (HA). HA is a glycosaminoglycan a chain of natural sugars distributed throughout human tissue that can accumulate around cancer cells. Halozyme has announced plans for rollout of a global phase 3 clinical study in 2016 targeting metastatic pancreatic cancer patients with high HA levels using its PEGPH20 in combination with ABRAXANE® (nab-paclitaxel) and gemcitabine.
Under the agreement, Ventana will develop an in vitro diagnostic (IVD), under design control, using Halozyme's proprietary HA binding protein, with the intent of submitting it for regulatory approval in the United States, Europe and other countries.
"Ventana brings a high level of development, regulatory and commercial expertise to our companion diagnostic strategy, which will help ensure we are well prepared for the initiation of our phase 3 study in pancreatic cancer," said Dr. Helen Torley, president and CEO of Halozyme. "The agreement is an important milestone in our PEGPH20 program as we study the potential of PEGPH20 across multiple tumor types."
"We are pleased to enter into this master collaboration agreement with Halozyme, which may produce the first diagnostic to target tumor-associated HA and possibly the first companion diagnostic assay in pancreatic cancer," said Doug Ward, Vice President, Ventana Companion Diagnostics. "The PEGPH20 program, coupled with our global reach, has the potential to improve the standard of care in pancreatic cancer for patients around the world."
The financial terms of the agreement were not disclosed.
This pharma collaboration is one of many at Ventana, where the Companion Diagnostics team is developing patient stratifying diagnostic tools that can help identify those individuals who are most likely to benefit from specific treatments.
Companion diagnostics (CDx) are tests designed to confirm the presence of a specific biomarker to assist physicians in selecting effective therapies for their patients, based on the individual characteristics of each person. Incorporating a companion diagnostic strategy into a drug development program may expedite the drug approval process and help generate more effective treatments with improved safety profiles for patients.
About Ventana Medical Systems, Inc.
Ventana Medical Systems, Inc. ("VMSI") (SIX: RO, ROG; OTCQX: RHHBY), a member of the Roche Group, innovates and manufactures instruments and reagents that automate tissue processing and slide staining for cancer diagnostics. VENTANA products are used in clinical histology and drug development research laboratories worldwide. The company's intuitive, integrated staining, workflow management platforms, and digital pathology solutions optimize laboratory efficiencies to help reduce errors, support diagnosis and enable informed treatment decisions by anatomic pathology professionals. Together with Roche, VMSI is driving Personalized Healthcare through accelerated drug discovery and the development of companion diagnostics to identify the patients most likely to respond favorably to specific therapies.
Visit www.ventana.com to learn more.
About PEGPH20
PEGPH20 is an investigational PEGylated form of Halozyme's proprietary recombinant human hyaluronidase under clinical development for the systemic treatment of tumors that accumulate hyaluronan.
The FDA granted orphan drug designation to PEGPH20 for treatment of pancreatic cancer and fast track for PEGPH20 in combination with gemcitabine and nab-paclitaxel for the treatment of metastatic pancreatic cancer. Additionally, the European Commission, acting on the recommendation from the Committee for Orphan Medicinal Products of the European Medicines Agency, designated investigational drug PEGPH20 an orphan medicinal product for the treatment of pancreatic cancer.
Clinical trials are currently ongoing for development of PEGPH20 in pancreatic ductal adenocarcinoma and in non-small cell lung cancer. More information may be found at: http://oncologytrials.halozyme.com/
About Halozyme
Halozyme Therapeutics is a biotechnology company focused on developing and commercializing novel oncology therapies that target the tumor microenvironment. Halozyme's lead proprietary program, our investigational drug PEGPH20, applies a unique approach to targeting solid tumors, allowing increased access of co-administered cancer drug therapies to the tumor. PEGPH20 is currently in development for metastatic pancreatic cancer and non-small cell lung cancer and has potential across additional cancers in combination with different types of cancer therapies. In addition to its proprietary product portfolio, Halozyme has established value-driving partnerships with leading pharmaceutical companies including Roche, Pfizer, Janssen and Baxter for its drug delivery platform, ENHANZE, which enables biologics and small molecule compounds that are currently administered intravenously to be delivered subcutaneously. Halozyme is headquartered in San Diego. For more information visit www.halozyme.com.
Safe Harbor Statement
In addition to historical information, the statements set forth above include forward-looking statements (including, without limitation, statements concerning the possible activity, benefits and attributes of PEGPH20, the possible method of action of PEGPH20, its potential application to improve cancer therapies and statements concerning future actions relating to the development of PEGPH20) that involve risk and uncertainties that could cause actual results to differ materially from those in the forward-looking statements. The forward-looking statements are typically, but not always, identified through use of the words "believe," "enable," "may," "will," "could," "intends," "estimate," "anticipate," "plan," "predict," "probable," "potential," "possible," "should," "continue," and other words of similar meaning. Actual results could differ materially from the expectations contained in forward-looking statements as a result of several factors, including unexpected expenditures and costs, unexpected results or delays in development and regulatory review, regulatory approval requirements, unexpected adverse events and competitive conditions. These and other factors that may result in differences are discussed in greater detail in the Company's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 11, 2015.
CONTACTS: | |
Halozyme Therapeutics | Ventana Medical Systems |
Schond Greenway | VMSI Media Relations |
858-704-8352 | Jacqueline Bucher |
Vice President, Marketing & | |
Corporate Communications | |
Jim Mazzola | 520-877-7288 o |
858-704-8122 | 520-468-9145 m |


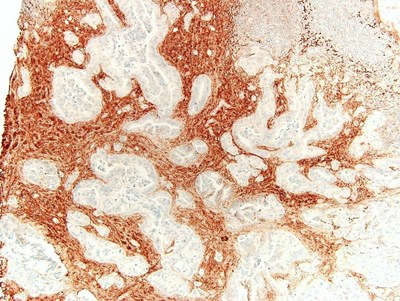
Hyaluronic Acid expressed in pancreatic cancer tissue at 40x magnification
Logo - http://photos.prnewswire.com/prnh/20100302/LA63139LOGO
Logo - http://photos.prnewswire.com/prnh/20150527/218705LOGO
Photo - http://photos.prnewswire.com/prnh/20150527/218706
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/halozyme-ventana-enter-into-global-agreement-to-collaboratively-develop-companion-diagnostic-for-cancer-treatment-300089204.html
SOURCE Halozyme Therapeutics, Inc.; Ventana Medical Systems, Inc.
 Help employers find you! Check out all the jobs and post your resume.
Help employers find you! Check out all the jobs and post your resume.




