Scott Sigman, M.D., today announced positive results from a randomized clinical pilot study for COVID-19 pneumonia patients treated with MLS Laser Therapy.
|
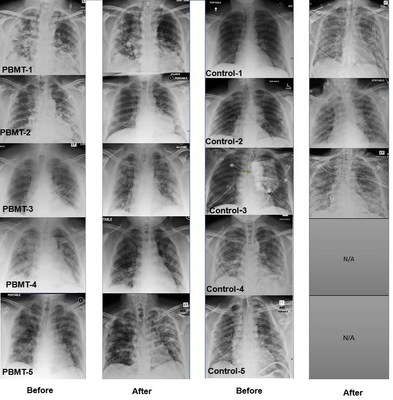
LOWELL, Mass., March 22, 2021 /PRNewswire/ -- Scott Sigman, M.D., today announced positive results from a randomized clinical pilot study for COVID-19 pneumonia patients treated with MLS Laser Therapy. Data published in the Journal of Inflammation Research showed patients who received adjunctive Photobiomodulation Therapy (PBMT) had an improvement of all evaluative indices and recovered quicker without ICU admission or mechanical ventilation.1 After a five-month follow-up, patients in the study who received PBMT were asymptomatic and experienced no long-term side effects. "PBMT is a non-invasive, non-pharmacotherapy and patients who received PBMT had a significant, rapid recovery," said Dr. Sigman. "Even with the COVID-19 vaccines, COVID-19 is not going away and there is a continued medical need for safe and effective COVID-19 treatment options. I hope the positive results from this study will encourage others to consider PBMT as an adjunct treatment option and support additional clinical trials with the use of PBMT." The study examined 10 patients with COVID-19 induced pneumonia treated with either standard medical care or standard medical care plus PBMT. The PBMT group received four once daily sessions of near-infrared light treatment targeting the lung tissue. Patient outcomes were measured via blood work; chest x-rays; pulse oximetry; and validated scoring tools for pneumonia, including SMART-COP score, which evaluates patients with severe pneumonia who need to be referred to ICU; BCRSS, which evaluates respiratory status of COVID-19 patients; CAP score to evaluate changes in symptoms and well-being before and after treatment; and RALE score to evaluate chest x-ray via glass opacity and consolidation in each lung. Results from the study also demonstrated PBMT-treated patients required less time to recover from COVID-19, with a mean hospital stay of 7 days. No ICU admissions or mechanical ventilation were required. These patients showed significantly improved RALE Chest X-ray score (from 8 to 5.2). Within 5–10 minutes of PBMT, the patients' SpO2 increased from 93–94% to 98–100% as detected by pulse oximetry. Improvements were seen in all other evaluative measures: SMART-COP (from 5.4 to 1.4), BCRSS (from 4.0 to 0.4), and CAP (from 41.5 to 82). In contrast, the control group patients, who received standard of care without PBMT, had a mean hospital stay of 12 days. 60% of these patients were admitted to the ICU for mechanical ventilation. RALE Chest X-ray scores worsened (from 4.4 to 6.2). In other evaluative tools: SMART-COP (from 2.8 to 4.4), BCRSS (from 2.4 to 3.6), and CAP (from 38.3 to 43.2). At a five-month follow-up, 40% of patients in the control group reported long-term effects. 40% of the patients in the control group did not survive. About PBMT The MLS laser, which is manufactured by ASA Laser based in Italy, uses a mobile scanner with 2 synchronized laser diodes, one in pulse mode (adjustable to 1–2000 Hz), emitting at 905 nm, and another in pulsed mode emitting at 808 nm. The two laser wavelengths work simultaneously and are synchronized. The laser is placed 20 cm above the patient across the lung fields while in the prone position. The laser is painless, and patients typically are unaware that the laser treatment is occurring. This laser is routinely used for deeper tissues like hips and pelvic joints that are surrounded by thick muscles. The therapeutic dose used to reach the deep targets of the pelvis is 4.5 J/cm2. Based on calculations by Dr. Soheila Mokmeli, co-author of the study, 7.2 J/cm2 was used over the skin, delivering a therapeutic dose just over 0.01 J/cm2 of laser energy to the lung. This dosage is able to penetrate the chest wall and reach the lung tissue creating an anti-inflammatory effect that in theory blocks the effects of the cytokine storm seen in COVID-19 pneumonia. For more information on MLS Laser Therapy, contact Mark Mollenkopf via email at mmollenkopf@celasers.com or telephone at 800-889-4184 Ext. 102. For more information about this preliminary work and study plans, contact Scott A. Sigman, M.D., via email at sasigmanmd@verizon.net or telephone at 978-856-7676. Media Contacts: Evan Wicker, Ph.D. 1 Vetrici MA, Mokmeli S, Bohm AR, Monici M, Sigman SA. Evaluation of Adjunctive Photobiomodulation (PBMT) for COVID-19 Pneumonia via Clinical Status and Pulmonary Severity Indices in a Preliminary Trial. J Inflamm Res. 2021;14:965-979.
SOURCE Cutting Edge Laser Technologies |