Analysis Shows Treatment Effect of Omecamtiv Mecarbil Increased Progressively as Baseline Ejection Fraction Decreased
Analysis Shows Treatment Effect of Omecamtiv Mecarbil Increased Progressively as Baseline Ejection Fraction Decreased
SOUTH SAN FRANCISCO, Calif., May 17, 2021 (GLOBE NEWSWIRE) -- Cytokinetics, Incorporated (Nasdaq: CYTK) today announced that data from a secondary analysis of GALACTIC-HF (Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure) assessing the effect of omecamtivmecarbil on clinical outcomes in relationship to patient baseline ejection fraction were presented by John Teerlink, M.D., Professor of Medicine, University of California San Francisco, Director of Heart Failure, San Francisco Veterans Affairs Medical Center and Executive Committee Chair, GALACTIC-HF in a Late Breaking Clinical Trial session at the American College of Cardiology 70th Annual Scientific Session & Expo (ACC.21) and were simultaneously published in the Journal of the American College of Cardiology.1
“These results build on our previous observation that the treatment effect of omecamtiv mecarbil grows progressively stronger in patients with lower ejection fraction, the very patients who are at the highest risk for heart failure hospitalization and cardiovascular death,” said John Teerlink, M.D. “Given that these severe heart failure patients are often unable to tolerate guideline-directed medical therapy and are left with few treatment options, omecamtiv mecarbil may provide an opportunity to improve their systolic function and keep them out of the hospital. In addition, omecamtiv mecarbil has no adverse effect on heart rate, blood pressure, kidney function or blood potassium levels, suggesting it can be added without interfering with guideline-directed medical therapy.”
“Despite excellent background therapy, heart failure patients are still in need of additional options as evidenced by the increasingly high event rates as baseline ejection fraction falls,” said Fady I. Malik, M.D., Ph.D., Cytokinetics’ Executive Vice President of Research & Development. “The data presented today underscore the potential value that omecamtiv mecarbil may have in reducing the clinical and economic burden of severe heart failure. We look forward to further analyses of GALACTIC-HF in the coming months and to advancing toward our goal of an NDA submission in the U.S. for omecamtiv mecarbil in the second half of 2021.”
GALACTIC-HF: Secondary Analysis
GALACTIC-HF enrolled 8,256 patients who were at risk of hospitalization and death, despite being well treated on standard of care therapy. As previously reported, after a median duration of follow-up of 21.8 months, the trial demonstrated a statistically significant effect of treatment with omecamtiv mecarbil to reduce risk of the primary composite endpoint of heart failure events (heart failure hospitalization and other urgent treatment for heart failure) or cardiovascular (CV) death compared to placebo in patients treated with standard of care (hazard ratio, 0.92; 95% confidence interval [CI] 0.86, 0.99; p=0.025). No reduction in the secondary endpoint of time to CV death was observed in the overall population. 1 Supplemental analyses indicated that the effect of omecamtiv mecarbil on the primary composite endpoint was consistent across most prespecified subgroups, with a progressively larger treatment effect of omecamtiv mecarbil with decreasing EF (interaction p = 0.004). This secondary analysis further investigates the influence of EF on the observed treatment effects.
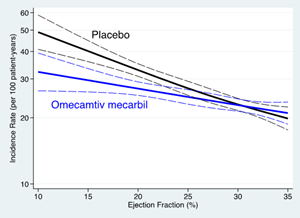
The analysis evaluated the effect of patient treatment with omecamtiv mecarbil based on quartiles of baseline EF defined as EF ≤22%, EF 23-28%, EF 29-32% and EF ≥33% as well as considering baseline EF as a continuous variable. The incidence of the primary outcome of first heart failure event or cardiovascular death increased with decreasing ejection fraction; in the lowest LVEF quartile (EF ≤22%) the incidence (35.6 per 100 patient-years) was almost 80% greater than in the highest EF quartile (EF ≥33%; 20 per 100 patient-years). Treatment with omecamtiv mecarbil demonstrated a 15% (HR 0.85; 95% CI 0.74-0.97; p = 0.016) and 17% (HR 0.83; 95% CI 0.73-0.95; p = 0.005) relative risk reduction in the lower two quartiles, respectively, compared to no difference in the upper two quartiles.
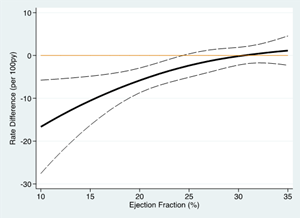
Analysis of ejection fraction as a continuous variable demonstrated a progressively larger treatment effect of omecamtiv mecarbil with decreasing ejection fraction (Figure A, interaction p = 0.013 by EF quartile). Accordingly, the absolute treatment effect on the primary composite endpoint also increased between the patients treated with placebo and omecamtiv mecarbil as baseline ejection fraction decreased (Figure B) such that in the lowest ejection fraction quartile, there was an absolute reduction of 7.4 events per 100 patient-years, with a number-needed-to-treat of 11.8 patients necessary to prevent an event over three years.
Figure A accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/1d595e71-7590-4988-8f2d-d3c5d1ca17ba
Figure B accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/1716ac6d-a536-44ab-86ae-f160660037ee
The beneficial effect of treatment with omecamtiv mecarbil on the primary composite endpoint was driven predominantly by the reduction in heart failure events. To determine the significance of EF subgroup differences, a test of the interaction effect revealed that EF was a significant modifier of this treatment effect (interaction p = 0.004 by EF quartile, interaction p = 0.001 by EF as continuous variable). Treatment with omecamtiv mecarbil demonstrated a 19% (HR 0.81; 95% CI 0.70-0.93) and 17% (HR 0.84; 95% CI 0.72-0.98) relative risk reduction in the lower two quartiles, respectively, compared to no difference in the upper two quartiles.
A greater reduction in NT-proBNP was also observed with omecamtiv mecarbil in patients with lower EF, with a 22% reduction (p < 0.001) in the lowest EF quartile, and a 3% reduction in the highest quartile (p = 0.54; interaction p < 0.001). NT-proBNP is a biomarker of ventricular wall stress, where higher levels reflect more severe heart failure.
As previously noted, treatment with omecamtiv mecarbil resulted in a small reduction in heart rate (treatment difference of 1.1 to 1.9 bpm across the EF quartiles) and increase in troponin I (median 3-5 ng/L across the EF quartiles; limit of detection, 6 ng/L; upper reference limit, 40 ng/L), though these results did not differ by EF quartile. There were no significant differences in systolic blood pressure, serum potassium, or creatinine or the incidence of adverse events between the omecamtiv mecarbil and placebo treated groups between EF quartiles.
GALACTIC-HF: Trial Design and Primary Results
GALACTIC-HF,2 (Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure), one of the largest Phase 3 global cardiovascular outcomes studies in heart failure ever conducted, enrolled 8,256 patients in 35 countries across 945 sites with HFrEF, New York Heart Association (NYHA) class II-IV, left ventricular ejection fraction (LVEF) ≤35%, elevated natriuretic peptides and either current hospitalization for heart failure or history of hospitalization or emergency department visit for heart failure within a year. Patients were randomized to either oral placebo or a starting dose of 25 mg omecamtiv mecarbil twice daily (maintenance dose of 50 mg, 37.5 mg, or 25 mg twice daily) guided by pharmacokinetic-guided dose selection. A blood test, the QMS Omecamtiv Mecarbil Immunoassay (the OM Test) was used to measure plasma levels of omecamtiv mecarbil in each patient in order to guide selection of the appropriate maintenance dose.
The primary composite endpoint of this double-blind, placebo-controlled, event-driven trial was time to CV death or first heart failure event (heart failure hospitalization and other urgent treatment for heart failure). Secondary endpoints were: time to CV death, patient reported outcomes (measured by Kansas City Cardiomyopathy Questionnaire [KCCQ] Total Symptom Score [TSS]), time to first heart failure hospitalization and time to all-cause death. A first primary endpoint event occurred in 1,523 of 4,120 patients (37.0%) in the omecamtiv mecarbil group and in 1,607 of 4,112 patients (39.1%) in the placebo group (hazard ratio, 0.92; 95% confidence interval [CI] 0.86, 0.99; p=0.025). No reduction in the secondary endpoint of time to CV death was observed in the overall population. The effect on the primary endpoint was observed without evidence of an increase in the overall rates of myocardial ischemic events, ventricular arrhythmias or death from cardiovascular or all causes.
About Omecamtiv Mecarbil and the Phase 3 Clinical Trials Program
Omecamtiv mecarbil is an investigational selective cardiac myosin activator, the first of a novel class of myotropes3 designed to directly target the contractile mechanisms of the heart, binding to and recruiting more cardiac myosin heads to interact with actin during systole. Preclinical research has shown that omecamtiv mecarbil increases cardiac contractility without increasing intracellular myocyte calcium concentrations or myocardial oxygen consumption.4-6 Cardiac myosin is the cytoskeletal motor protein in the cardiac muscle cell that is directly responsible for converting chemical energy into the mechanical force resulting in cardiac contraction.
Omecamtiv mecarbil is being developed for the potential treatment of heart failure with reduced ejection fraction (HFrEF) and is the subject of a comprehensive Phase 3 clinical trials program composed of GALACTIC-HF and METEORIC-HF (Multicenter Exercise Tolerance Evaluation of Omecamtiv Mecarbil Related to Increased Contractility in Heart Failure), a Phase 3 clinical trial designed to evaluate the effect of treatment with omecamtiv mecarbil compared to placebo on exercise capacity.
About Heart Failure
Heart failure is a grievous condition that affects more than 64 million people worldwide7 about half of whom have reduced left ventricular function.8,9 It is the leading cause of hospitalization and readmission in people age 65 and older.10, 11 Despite broad use of standard treatments and advances in care, the prognosis for patients with heart failure is poor.12 An estimated one in five people over the age of 40 are at risk of developing heart failure, and approximately 50 percent of people diagnosed with heart failure will die within five years of initial hospitalization.13,14 More than 2 million people in the U.S. are estimated to have an ejection fraction <30%, indicating they may have severe heart failure.15
About Cytokinetics
Cytokinetics is a late-stage biopharmaceutical company focused on discovering, developing and commercializing first-in-class muscle activators and next-in-class muscle inhibitors as potential treatments for debilitating diseases in which muscle performance is compromised and/or declining. As a leader in muscle biology and the mechanics of muscle performance, the company is developing small molecule drug candidates specifically engineered to impact muscle function and contractility. Cytokinetics is engaging with regulatory authorities in preparation for a U.S. NDA submission of omecamtiv mecarbil, its novel cardiac muscle activator, following positive results from GALACTIC-HF, a large, international Phase 3 clinical trial in patients with heart failure. Cytokinetics is conducting METEORIC-HF, a second Phase 3 clinical trial of omecamtiv mecarbil. Cytokinetics is also developing CK-274, a next-generation cardiac myosin inhibitor, for the potential treatment of hypertrophic cardiomyopathies (HCM). Cytokinetics is conducting REDWOOD-HCM, a Phase 2 clinical trial of CK-274 in patients with obstructive HCM. Cytokinetics is also developing reldesemtiv, a fast skeletal muscle troponin activator for the potential treatment of ALS and other neuromuscular indications following conduct of FORTITUDE-ALS and other Phase 2 clinical trials. The company is preparing for the potential advancement of reldesemtiv to a Phase 3 clinical trial in ALS. Cytokinetics continues its over 20-year history of pioneering innovation in muscle biology and related pharmacology focused to diseases of muscle dysfunction and conditions of muscle weakness.
For additional information about Cytokinetics, visit www.cytokinetics.com and follow us on Twitter, LinkedIn, Facebook and YouTube.
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the "Act"). Cytokinetics disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act's Safe Harbor for forward-looking statements. Examples of such statements include, but are not limited to, the potential benefits of omecamtiv mecarbil, including its ability to represent a novel therapeutic strategy to increase cardiac muscle function and restore cardiac performance; the timing and likelihood of any regulatory submissions or approval of omecamtiv mecarbil, Cytokinetics' research and development activities; the design, timing, results, significance and utility of preclinical and clinical results; and the properties and potential benefits of Cytokinetics' other drug candidates. Such statements are based on management's current expectations, but actual results may differ materially due to various risks and uncertainties, including, but not limited to, potential difficulties or delays in the development, testing, regulatory approvals for trial commencement, progression or product sale or manufacturing, or production of Cytokinetics' drug candidates that could slow or prevent clinical development or product approval; Cytokinetics' drug candidates may have adverse side effects or inadequate therapeutic efficacy; the FDA or foreign regulatory agencies may delay or limit Cytokinetics' ability to conduct clinical trials; Cytokinetics may be unable to obtain or maintain patent or trade secret protection for its intellectual property; standards of care may change, rendering Cytokinetics' drug candidates obsolete; and competitive products or alternative therapies may be developed by others for the treatment of indications Cytokinetics' drug candidates and potential drug candidates may target. For further information regarding these and other risks related to Cytokinetics' business, investors should consult Cytokinetics' filings with the Securities and Exchange Commission.
Contact:
Cytokinetics
Diane Weiser
Senior Vice President, Corporate Communications, Investor Relations
(415) 290-7757
References
- Teerlink JR., Diaz R., Felker GM., et al. Effect of Ejection Fraction on Clinical Outcomes in Patients treated with Omecamtiv Mecarbil in GALACTIC-HF. JACC. 2021
- Teerlink JR., Diaz R., Felker GM., et al. Omecamtiv Mecarbil in Chronic Heart Failure With Reduced Ejection Fraction: Rationale and Design of GALACTIC-HF. JACC Heart Fail. 2020 Apr; 8(4):329-340. doi: 10.1016/j.jchf.2019.12.001.Epub 2020 Feb 6.
- Psotka MA, Gottlieb SS, Francis GS et al. Cardiac Calcitropes, Myotropes, and Mitotropes. JACC. 2019; 73:2345-53.
- Planelles-Herrero VJ, Hartman JJ, Robert-Paganin J. et al. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat Commun. 2017;8:190.
- Shen YT, Malik FI, Zhao X, et al. Improvement of cardiac function by a cardiac myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010; 3: 522-27.
- Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011 Mar 18;331(6023):1439-43.
- James et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Lancet 2018; 392: 1789–858.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200.
- Roger VL. Epidemiology of Heart Failure. Circulation Research. 2013;113:646-659, originally published August 29, 2013. Doi: 10.1161/CIRCRESAHA.113.300268.
- Kilgore M, Patel HK, Kielhorn A et al. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017; 10: 63-70.
- Jhund PS, MacIntyre K, Simpson CR, et al. Long-Term Trends in First Hospitalization for Heart Failure and Subsequent Survival Between 1986 and 2003. Circulation. 2009;119:515-523.
- Benjamin EJ, Virani SS, Callaway CW et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492.
- Roger VL, Weston SA, Redfield MM, et al. Trends in Heart Failure Incidence and Survival in a Community-Based Population. JAMA. 2004;292:344-350.
- Shannon M. Dunlay, Véronique L. Roger, Susan A. Weston, Ruoxiang Jiang, and Margaret M. Redfield (Circ Heart Fail. 2012;5:720-726.); Olmsted County community cohort of HF patients (1984 to 2009).