SCOTTSDALE, Ariz., Aug. 11, 2015 /PRNewswire/ -- CyMedica Orthopedics, Inc. today announced that it has received a CE (Conformité Européenne) Mark for its QB1 Muscle Activation System. The product received its FDA 510(k) clearance in April 2015, and has since enjoyed a tremendous reception from orthopedic surgeons and knee patients across the United States.
"Securing the CE Mark for the QB1 System marks an important milestone for our company, greatly expanding our market opportunity to patients in Europe and paving the way for a global sales and distribution network. We are excited to have the clearance to deliver our revolutionary technology to millions of patients outside of the United States," said Rob Morocco, President and CEO of CyMedica Orthopedics. "Having recently launched domestically, we will continue to dedicate ourselves to expanding our market penetration within the US as we evaluate the timing and structure of our plans to commercialize the QB1 internationally."
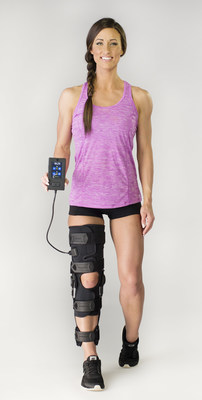
The CE mark opens new international markets to the QB1 to benefit patients suffering from quadriceps muscle atrophy a weakening of the major leg muscles after ACL and total knee replacement surgery. Quad atrophy can significantly slow healing and affect a patient's activities, leaving them prone to delayed rehabilitation and further injury.
CyMedica Orthopedics brought together leading orthopedic surgeons, physical therapists and engineering experts to deliver the first Muscle Activation System that activates atrophied quad muscles in a clinically relevant manner with little pain. The QB1 incorporates this patented and intelligent technology with a postoperative knee brace to bring rehabilitation therapy into a patient's home. This enables all knee patients to potentially improve their quad strength markedly faster and more comfortably than with other methods. Patients can now receive the same frequent, consistent, professional treatment previously reserved for elite athletes.
About CyMedica Orthopedics, Inc. Based in Scottsdale, Arizona, CyMedica Orthopedics is a privately held medical device company that develops and commercializes innovative products that target muscle atrophy using its platform technology. That technology includes the first closed-loop power control system to provide patients comfortable yet aggressive treatment.
For more information on the CyMedica QB1 please visit www.cymedicaortho.com
Photo - http://photos.prnewswire.com/prnh/20150810/257359
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/cymedica-orthopedics-receives-ce-mark-for-the-cymedica-qb1-muscle-activation-system-300126491.html
SOURCE CyMedica Orthopedics, Inc.
 Help employers find you! Check out all the jobs and post your resume.
Help employers find you! Check out all the jobs and post your resume.




