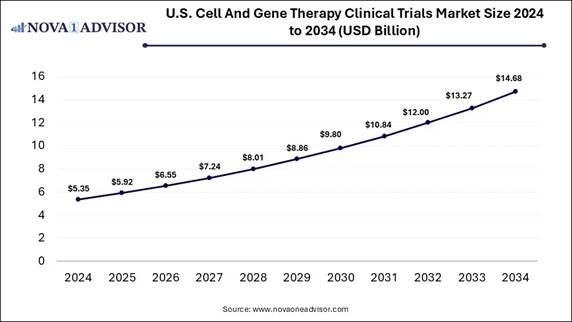
According to Nova One Advisor, the U.S. cell and gene therapy clinical trials market size is expected to be worth around 14.68 billion by 2034, increasing from USD 5.92 billion in 2025, representing a healthy CAGR of 15.62% from 2025 to 2034.
The U.S. cell and gene therapy clinical trials market is expanding as it provides many advantages such as targets the root cause, treating previously incurable diseases, a one-time treatment approach, potential for long-lasting effects, and providing potential for personalized medicine. Gene therapy is used to lessen levels of a disease-causing type of protein, increase production of disease-fighting proteins, or produce modified proteins.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/9195
Approved Cell and Gene Therapies Products in 2025
|
Product & Trade Name |
Manufacturer |
|
ABECMA (idecabtagene vicleucel) |
Celgene Corporation, a Bristol-Myers Squibb Company |
|
ADSTILADRIN (nadofaragene firadenovec-vcng) |
Ferring Pharmaceuticals A/S |
|
ALLOCORD (HPC, Cord Blood) |
SSM Cardinal Glennon Children's Medical Center |
|
AMTAGVI (lifileucel) |
Iovance Biotherapeutics, Inc. |
|
AUCATZYL (obecabtagene autoleucel) |
Autolus Limited |
|
BEQVEZ (fidanacogene elaparvovec-dzkt) |
Pfizer, Inc. |
|
CARVYKTI (ciltacabtagene autoleucel) |
Janssen Biotech, Inc. |
U.S. Cell and Gene Therapy Clinical
Trials Market Highlights: • By phase, the phase III segment dominated
the market with the largest share in 2024. • By phase, the phase I segment is expected
to show the fastest growth over the forecast period. • By indication, the oncology segment held
the largest market share in 2024. • By indication, the cardiology segment is
expected to register fastest growth during the forecast period. Market Overview and Industry Potential Gene
and cell therapy clinical trials are use structured as a phase I / II
study where a small group of contributors with the disease are registered and
both efficacy and safety tests are performed. They required adequate time to
fully assess the results of a trial whether advantages outweigh risks.
Contributing in clinical
trials is an opportunity to receive a new product, while moving along
research to supports others who have the same disease or condition. The
clinical study is led in a group of people with a precise disease or condition
to test the drug's efficacy and safety. Gene and cell therapy provide an
opportunity to fix those genetic errors who are impossible to treat in past. In
this trial, a physician transplants human cells in a patient to replace or
repair damaged cells. Cell and gene therapy become real for patients when
innovations translate into the manufacture of advanced therapy medicinal
products (ATMPs). These novel therapies treat genetic diseases at the source by
moving changes in their cells, tissue, or DNA
with the significant to offer an efficient ‘once only’ treatment to ease severe
disease. As these diseases are caused on a genetic level, they were
conventionally understood poorly and largely considered hopeless. Genetic
diseases continue prevalent, with many having few, if any, treatments to
alleviate indications. Latest Trends of the Market ⬥︎ In May 2025, Genprex, Inc., a clinical-stage gene
therapy company focused on developing life-changing therapies for patients with
cancer and diabetes, announced that it has been selected to present at the upcoming
2025 American Society of Clinical Oncology (ASCO). ⬥︎ In May 2025, AviadoBio, a pioneering gene therapy
company devoted to developing and delivering potentially transformative
medicines for neurodegenerative disorders, announced that its Phase 1/2
ASPIRE-FTD clinical trial is open in the UK. The trial is appraising AVB-101,
an new gene therapy, in people with frontotemporal dementia (FTD) with GRN gene
mutations (FTD-GRN). Cambridge University Hospitals NHS Foundation Trust (CUH),
which hosts an internationally renowned centre of excellence in offering
support and care for families affected by FTD, is present recruiting patients. ⬥︎ In June 2025, the CGTLive team was
diligently tracking the FDA's activities related to the development of cell and
gene therapies for the treatment of rare, complex, and otherwise challenging
diseases and disorders. The agency has continued to ramp up its activities
around these therapies as more of them progress through the pipeline in tandem. Recent Advancements of Cell and Gene
Therapy Clinical Trials for Drug Discovery: Market’s Largest Potential Increasing cutting edge technology of cell
and gene therapies (CGTs) in drug discovery creates the opportunity to the
growth of the market. It is most advanced medical treatments, precisely
designed to correct the root genetic cause of a fundamental health challenge.
CGTs have the strength to transform conventional treatment for a broad range of
diseases that exhibit poor efficiesncy. Initiation and advancements in gene editing
devices and cell therapy srtratergies, including autologous chimeric antigen receptor
(CAR) T cell therapy, CRISPR-Cas9,
and viral vector engineering strategies, have expressively contributed to huge
growth in CGTs sectior. Automation platforms allow consistent large-scale and
small-scale production, which suggestively involve to the controlling of
manufacturing expenses. ⬥︎ For Instance, In July 2025, Atsena Therapeutics, a clinical-stage gene
therapy company focused on using the life-changing power of genetic
medicine to reverse or prevent blindness, announced that the U.S. Food and Drug
Administration (FDA) has agreed to the expansion of the company’s ongoing Phase
1 / 2 LIGHTHOUSE study of ATSN-201 into a continuous Phase 1 / 2 / 3 trial,
enabling it to serve as a pivotal trial to support a Biologics License
Application (BLA) submission for the treatment of X-linked retinoschisis
(XLRS). Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/9195
Report Scope of U.S. Cell And Gene
Therapy Clinical Trials Market
Report Coverage Details Market Size in 2025 USD 5.92 Billion Market Size by 2034 USD 14.68 Billion Growth Rate From 2025
to 2034 CAGR of 10.62% Base Year 2024 Forecast Period 2025-2034 Segments Covered Phase, Indication Market Analysis
(Terms Used) Value (US$ Million/Billion)
or (Volume/Units) Key Companies
Profiled Charles River
Laboratories, ICON Plc, IQVIA, LabCorp, Medpace, Novotech, PAREXEL
International Corp.,Syneos Health, Thermo Fisher Scientific, Inc., Veristat,
LLC
Country Level Analysis: Increasing approved gene therapy treatments
available in the United States drive the growth of the market. Leading research
and developing a safe and effective gene therapy is expensive, and it can be
challenging and inefficient to produce a gene therapy on a large scale. More
than 4,000 gene, cell, and RNA therapies in expansion. Early–phase clinical
trials are significant for evaluating novel medicine safety, tolerability, and
pharmacokinetics. They offer appreciated insights in the
safety of potential therapeutic agents before they are progressive to
larger–scale research. The cell and gene therapy areas are increasing
exponentially, with novel players developing daily and much development being
made both in and out of the laboratory. ⬥︎ For Instance, In April 2025, Abeona
Therapeutics Inc. announced the U.S. Food and Drug Administration (FDA) has
approved ZEVASKYN gene-modified cellular sheets, also known as pz-cel, as the
first and only autologous cell-based gene therapy for the treatment of wounds in
adult and pediatric patients with recessive dystrophic epidermolysis bullosa
(RDEB), a serious and debilitating genetic skin disease. U.S. Cell and Gene Therapy Clinical
Trials Market Segmentation Analysis: By Phase Analysis: The phase III segment dominates in the U.S.
cell and gene therapy clinical trials market, as phase III trial continues with
the emphasis on safety and efficacy, but increases the standards than the Phase
II by growing the size and refining the endpoints essential for approval. A
drug in Phase III can be under investigation for many years and potentially
enrol thousands of subjects in a randomized, double-blind, placebo-controlled
trial. The NDA covers all the scientific information that the business has
gathered during the phases in all trials. On the other hand, the phase I segment is
expected to grow exponentially during the forecast period as phase I trials are
done to discover the highest dose of a novel treatment that can be given safely
deprived of causing serious adverse effects. These studies help to decide on
the greatest way to give the novel treatment. Phase I trials look at what the
treatment does to the body and what the body does with the treatment. Phase one
in most clinical trials tests if the therapy is safe for a small number of well
volunteers. In gene and cell therapy clinical trials, the conduct can be far
more challenging and very precise to a disease, so strong participants are not
generally used. By Indication Analysis: The oncology
segment generated the highest market revenue in 2024, as clinical
trials are the novel ways to prevent, find, and treat cancer. They support
physician to improve the quality of life for patients with cancer by testing
ways to manage the adverse effects of cancer and its treatment. Most cancer
clinical trials are conduct studies that contain people who have cancer. These
trials test novel treatments or new ways of using present treatments. The main
aim of cancer screening trials is to test ways to find cancer before it causes
symptoms, when it may be easier to manage. On the other hand, the cardiology segment
is expected to grow exponentially during the forecast period as therapeutic use
of cell therapies has the probable to reverse myocardial injury and enhance
cardiac function, in contrast to most present medical therapies. Cell-based
therapy enhanced quality of life for patients with progressive heart failure,
Mayo Clinic scientists and international partners discovered in a late-stage
international clinical trial. Some of the Prominent Players in the
U.S. Cell and Gene Therapy Clinical Trials Market • ICON
Plc • IQVIA • LabCorp • Medpace • Novotech • PAREXEL International Corp. • Thermo Fisher
Scientific, Inc. • Veristat, LLC What is Going Around the Globe? ⬥︎ In April 2025, INmune Bio Inc. a clinical-stage
biotechnology company targeting inflammation and immunology through the innate
immune system has partnered with the Cell and Gene Therapy Catapult (CGT
Catapult) to establish large-scale, commercial-ready manufacturing for its cell
therapy platforms. CGT Catapult is an independent technology and innovation
organization specializing in the advancement of the cell and gene therapy
industry. ⬥︎ In April 2025, AGC Biologics launched
New Dedicated Cell and Gene Business Division. The new Cell and Gene
Technologies Division will focus on elevating existing AGC Biologics
capabilities and supporting developers in need of capacity, scientific
capabilities, and technically qualified cell and gene CDMO operators You can place an order or ask any
questions, please feel free to contact at sales@novaoneadvisor.com |
+1 804 441 9344 Related Report – ⬥︎ Cell And Gene Therapy Clinical Trials Market - https://www.novaoneadvisor.com/report/cell-and-gene-therapy-clinical-trials-market
⬥︎ Cell and Gene Therapy Market - https://www.novaoneadvisor.com/report/cell-and-gene-therapy-market
⬥︎ U.S. Cell And Gene Therapy Clinical Trial Services Market - https://www.novaoneadvisor.com/report/us-cell-gene-therapy-clinical-trial-services-market
⬥︎ Cell & Gene Therapy Contract Research Organizations Market - https://www.novaoneadvisor.com/report/cell-and-gene-therapy-contract-research-organizations-market
⬥︎ Cell And Gene Therapy Bioanalytical Testing Services Market - https://www.novaoneadvisor.com/report/cell-and-gene-therapy-bioanalytical-testing-services-market
⬥︎ Cancer Gene Therapy Market - https://www.novaoneadvisor.com/report/cancer-gene-therapy-market
⬥︎ Cell And Gene Therapy Manufacturing Market - https://www.novaoneadvisor.com/report/cell-and-gene-therapy-manufacturing-market
⬥︎ U.S. Gene Therapy Market - https://www.novaoneadvisor.com/report/us-gene-therapy-market
⬥︎ U.S. Cell And Gene Therapy Manufacturing Market - https://www.novaoneadvisor.com/report/us-cell-and-gene-therapy-manufacturing-market
Segments Covered in the Report This report forecasts revenue growth at
country levels and provides an analysis of the latest industry trends in each
of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc.
has segmented the U.S. cell and gene therapy clinical trials market. By Phase • Phase I • Phase II • Phase III • Phase IV By Indication • Oncology • Cardiology • CNS • Musculoskeletal • Infectious diseases • Dermatology • Endocrine, metabolic, genetic • Immunology & inflammation • Ophthalmology • Hematology • Gastroenterology • Others Immediate Delivery Available | Buy This
Premium Research https://www.novaoneadvisor.com/report/checkout/9195
About-Us Nova One Advisor is a global leader
in market intelligence and strategic consulting, committed to delivering deep,
data-driven insights that power innovation and transformation across
industries. With a sharp focus on the evolving landscape of life sciences, we
specialize in navigating the complexities of cell and gene therapy, drug
development, and the oncology market, enabling our clients to lead in some of
the most revolutionary and high-impact areas of healthcare. Our expertise spans the entire
biotech and pharmaceutical value chain, empowering startups, global
enterprises, investors, and research institutions that are pioneering the next
generation of therapies in regenerative medicine, oncology, and precision medicine.
Web: https://www.novaoneadvisor.com/ Contact Us USA: +1 804 420 9370 Email: sales@novaoneadvisor.com For Latest Update
Follow Us: LinkedIn

