In AI-driven drug discovery, artificial intelligence has been widely applied across multiple stages of R&D, including target identification, virtual screening, molecular generation, and synthesis route planning. However, in real-world drug design—particularly when addressing first-in-class targets, difficult-to-drug targets such as pan-mutant KRAS, or other settings where microscopic-level research information are limited—existing AI methods continue to face clear limitations. By contrast, quantum computing is based on first-principles approaches and simulates molecular systems by following the laws of quantum mechanics at the microscopic scale, providing drug discovery teams with an alternative technical pathway at the level of fundamental molecular information.
Quantum computing has demonstrated significant research value in the biomedical field, yet the transition from technical exploration to practical application continues to face a critical challenge: the lack of a unified and comparable evaluation standard. At present, core quantum algorithms—most notably the variational quantum eigensolver (VQE)—have primarily been applied to small reference molecular systems, such as H₂, H₂O, LiH, and BeH₂, or have been limited to case-specific studies involving individual drug-like compounds. By contrast, there remains no widely adopted benchmark framework capable of systematically evaluating the performance of different quantum algorithms and hardware configurations at the scale of realistic drug-like molecules.
Recently, the world’s first benchmark for quantum computing–based drug design was released. The benchmark was jointly developed by QureGenAI together with China Pharmaceutical University, Ningbo University of Technology and Hun-Dun Quantum Experimental Platform of China Mobile (Benchmark link: https://arxiv.org/abs/2512.18203). Designed for practical application scenarios in real world drug discovery, the benchmark provides open-source code and test datasets and aims to offer a fair and comparable evaluation framework to support the continued iteration of quantum computing applications in biology and pharmaceutical industry, as well as for cross-platform comparisons across different quantum hardware approaches.
The core innovation of the benchmark lies in the establishment, for the first time, of a standardized evaluation framework that connects quantum algorithms with realistic drug-like molecules. For drug discovery researchers, its immediate value lies in being readily usable in practice and in reducing the need for extensive empirical exploration.
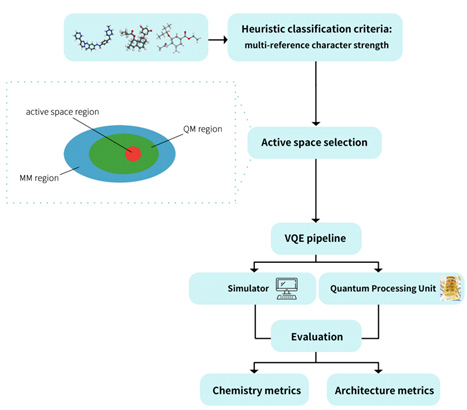
VQE benchmark workflow of QureGenAI
This work establishes the first systematic VQE benchmarking framework driven by active space selection. At the methodological level, the benchmark defines a clear set of evaluation criteria, including heuristic classification based on chemically grounded metrics , diverse molecular benchmark suite—such as lovastatin and oseltamivir, employ both UCCSD (unitary coupled-cluster with singles and doubles) and HEA (hardware-efficient ansatz) ansatze and it adopts a multi-dimensional evaluation that integrates both chemistry metrics and architecture metrics. Most importantly, by performing computations on real superconducting quantum processing unit (QPU), the benchmark achieves a critical step from simulation-based validation to empirical evaluation.
The research team performed hardware-level validation on two superconducting quantum processors with different qubit counts, using three representative molecules (H₂O, aspirin, and benzene). The experiments evaluated two superconducting quantum processors with different numbers of qubits, two basis sets (STO-3G and 6-31G(d)), and a two-qubit hardware-efficient ansatz (HEA). Across these settings, the resulting converged energy values exhibited stable convergence behavior. These results are essential for assessing the practical relevance of quantum computing in drug discovery and provide empirical evidence supporting hardware–algorithm co-design.
The release of the benchmark addresses key practical application pain points in quantum drug discovery, providing pharmaceutical companies and research organizations with technical guidance that can be directly applied. Based on this benchmark, drug development teams can more efficiently identify VQE parameter configurations aligned with their specific needs, thereby reducing repeated trial-and-error costs in parameter selection and experimental setup.
Similarly, the establishment of the benchmark helps advance the standardization of technologies in the field of Quantum AIDD, enabling result comparison and collaboration across different research teams and companies, and thereby improving the efficiency of technology iteration. In addition, its open-source design lowers the barrier to applying quantum computing in drug discovery, allowing more organizations to pursue research based on a validated benchmark without starting from low-level parameter exploration.
Overall, as quantum hardware performance continues to improve and benchmark frameworks of this kind are further refined, quantum computing is expected to develop closer synergies with AI-driven drug discovery in the future. Such synergy may, to a certain extent, help alleviate long-standing challenges in traditional drug discovery—including limitations in precision and the scarcity of high-quality data—while exploring new technical pathways that could potentially support gains in drug R&D efficiency.
About QureGenAI
QureGenAI is a drug discovery company operating under a drug pipeline development and licensing business model, integrating quantum computing and AI technologies in its R&D approach(aka Quantum AIDD). The company currently has nine first-in-class (FIC) assets in development, including two at the Pre-IND stage and four at the PCC stage. Its research focus spans multiple therapeutic areas, including novel targets for androgenetic alopecia (AGA), pan-KRAS inhibitors, and HIF-2α agonists.
