According to Coherent Market Insights the global In-Vitro Diagnostics Market is estimated to be valued at USD 126.73 Bn in 2025 and is expected to reach USD 197.06 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.5% from 2025 to 2032. The global In Vitro Diagnostics (IVD) market is experiencing steady growth, driven by rising prevalence of chronic and infectious diseases, increasing adoption of personalized medicine, and advancements in diagnostic technologies such as molecular testing, next-generation sequencing, and point-of-care solutions. Growing demand for early and accurate disease detection, coupled with expanding healthcare infrastructure in emerging markets, is creating significant opportunities for industry players.
Request Sample Report: https://www.coherentmarketinsights.com/insight/request-sample/195
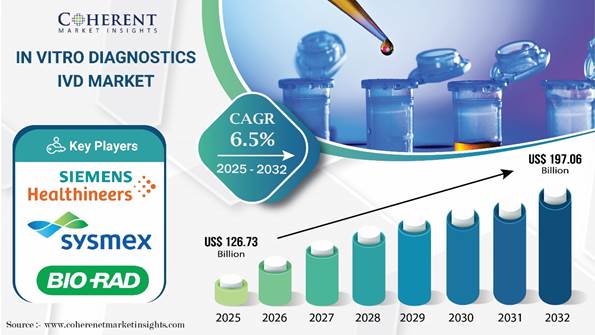
Global In Vitro Diagnostics (IVD) Market Key Takeaways
According to Coherent Market Insights (CMI), the global in vitro diagnostic IVD market size is projected to total USD 126.73 Bn in 2025 and further grow at a CAGR of 6.5%, surpassing USD 197.06 Bn by 2032.
Reagents & kits are expected to remain top-selling products, accounting for a market revenue of around USD 86.55 Bn in 2025.
By test type, immunoassay category is expected to hold a 34.6% share in the global in-vitro diagnostics (IVD) market by 2025.
Based on application, infectious diseases segment is set to account for nearly half of the global in vitro diagnostics IVD market share in 2025.
North America is likely to retain its dominance during the forecast period, holding a global industry share of 44.3% in 2025. This is attributable to growing focus on early disease detection and prevention and strong presence of leading in-vitro diagnostics companies.
As per CMI’s new in vitro diagnostics IVD market analysis, Asia Pacific is projected to experience fastest growth over the assessment period.
Immediate Delivery Available | Buy This Premium Research Report: https://www.coherentmarketinsights.com/insight/buy-now/195
Rising Incidence of Chronic and Infectious Diseases Spearheading Market Growth
Coherent Market Insights’ latest in vitro diagnostics IVD market research report offers insights into key factors driving market growth. One such major growth driver is the rising prevalence of chronic and infectious diseases.
There is a spike in chronic conditions like cancer, diabetes, and cardiovascular disease. This is expected to drive demand for in-vitro diagnostic tests as these diseases require timely, accurate, and rapid diagnostic testing.
Similarly, the looming threat of infectious diseases like COVID-19, influenza, and HIV will play a key role in fostering growth of the in-vitro diagnostics market. These diagnostic tests are crucial for early detection, disease surveillance, and effective management of chronic and infectious diseases.
Also Read: In Vitro Toxicity Testing Market Size, Share & Trend Analysis Report for 2025-2032
Regulatory Hurdles and High Cost Restraining In-Vitro Diagnostics Market Growth
The prospective in-vitro diagnostics (IVD) market outlook indicates steady growth. However, high cost of advanced IVD tests and regulatory challenges may limit market growth to some extent during the forthcoming period.
Next-generation sequencing, Molecular diagnostics, and genetic testing can be expensive. This high cost deters their adoption, especially in low and middle-income regions, dampening overall in vitro diagnostics IVD market demand.
Furthermore, in-vitro diagnostic tests are subject to stringent regulatory approval processes. These regulatory challenges may delay product launches and increase compliance costs, thereby negatively impacting the in-vitro diagnostics (IVD) market growth.
Advancements in Diagnostic Technologies Creating Growth Opportunities
Growing need for advanced diagnostics is triggering a wave of innovation in molecular diagnostics, NGS, point-of-care (POC) testing, and AI-integrated diagnostics. These advancements are improving test speed, accuracy, and accessibility, unlocking new growth opportunities for in vitro diagnostics IVD companies.
Impact of AI on the In Vitro Diagnostics (IVD) Market
Artificial intelligence (AI) is revolutionizing the in-vitro diagnostics market. It enhances the speed, accuracy, and efficiency of diagnostic procedures by enabling advanced data analysis and automation.
AI-powered tools enable rapid data analysis from complex diagnostic tests. This leads to earlier disease detection and more personalized treatment strategies. Machine learning algorithms are improving image interpretation in pathology and radiology.
Similarly, AI integration with lab automation is streamlining workflows and reducing human error. These technological advancements are not only optimizing clinical decision-making but also driving the development of predictive diagnostics, enabling proactive healthcare management.
Request For Customization: https://www.coherentmarketinsights.com/insight/request-customization/195
Emerging In Vitro Diagnostics (IVD) Market Trends
Growing demand for personalized medicine is a key growth-shaping trend in the in vitro diagnostics market. As healthcare moves toward tailored treatment approaches, there is increasing reliance on companion diagnostics and biomarker-based testing, especially in areas like oncology and genetic disorders.
Rising geriatric population is expected to uplift in vitro diagnostics demand during the forecast period. This is because older people are more prone to chronic diseases like arthritis, cardiovascular conditions, and Alzheimer’s disease, leading to increased need for diagnostic testing.
Increasing interest in preventive healthcare is expected to boost in vitro diagnostics IVD market value during the projection period. Governments and healthcare organizations are increasingly launching awareness campaigns and early disease detection initiatives. These efforts are likely to accelerate the adoption of IVD tests.
There is also a growing trend towards performing diagnostic tests in home care settings due to increased convenience, accessibility, and patient empowerment. This shift towards home-based testing will contribute to expansion of the in vitro diagnostics (IVD) market.
Also Read: Rapid Diagnostics Market Analysis and Forecast for 2025-2032
Analyst’s View
“The global in vitro diagnostics (IVD) industry is expected to grow steadily, owing to rising burden of chronic and infectious diseases, increasing focus on early detection and preventive healthcare, and continuous advancements in diagnostic technologies,” said Komal Dighe, a senior analyst at CMI.
Current Events and Their Impact on the In Vitro Diagnostics (IVD) Market
|
Event |
Description and Impact |
|
AI Integration in IVD with Real-World Clinical Use |
|
|
Post-Pandemic Global Health Security Push (2024–2025) |
|
|
Aging Populations and Precision Medicine in G20 Nations |
|
Competitor Insights
Key companies listed in the in-vitro diagnostics (IVD) market report include:
- Sysmex Corporation
- Siemens Healthineers
- Bio-Rad Laboratories
- Becton Dickinson and Company
- bioMérieux S.A.
- Abbott Laboratories
- F. Hoffmann-la Roche Ltd
- Danaher Corporation
- Arkray Inc.
- QIAGEN N.V.
- Medical & Biological Laboratories Co. Ltd.
- Nittobo Medical Co. Ltd.
- Mizuho Medy Co. Ltd.
- Miraca Holdings Inc.
- Diasorin
- Thermo Fischer Scientific Inc.
- Grifols SA
- Agilent Technologies, Inc.
Key Developments
In May 2025, the United States Food and Drug Administration (FDA) approved Fujirebio Diagnostics’ Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio test for diagnosing Alzheimer’s disease. It is the first-ever in vitro diagnostic blood test authorized in the United States to assist in diagnosing Alzheimer’s disease.
In February 2025, bioMerieux introduced GENR-UP TYPER, a novel diagnostic solution for rapid root cause analysis in the food industry. This automated system offers a cutting-edge solution to pathogen detection with its high speed, precision, and ease of use.
In September 2024, Sysmex Corporation expanded its diagnostics portfolio with the launch of HISCL HIT IgG Assay kit. It is designed to measure IgG-class antibodies against platelet factor 4–heparin (PF4–heparin) complexes, which are associated with heparin‑induced thrombocytopenia (HIT).
In April 2024, Grifols’ Procleix ArboPlex Assay obtained CE mark under the In Vitro Diagnostic Regulation (IVDR).
Also Read: In Vitro Fertilization Devices Market Size, Share, Trends & Opportunities for 2025-2032
Market Segmentation
- Product Type:
- Reagents & Kits
- Instruments
- Test Type:
- Clinical Chemistry
- Immunoassay
- Hematology
- Molecular Diagnostics
- Microbiology
- Coagulation & Hemostasis Urinalysis
- Others
- Application:
- Infectious Diseases
- Diabetes
- Oncology
- Cardiology
- Nephrology
- Autoimmune Disorders
- Others
- End User:
- Hospitals & Clinics
- Diagnostic Laboratories
- Academic & Research Institutes
- Others
- Region:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
Our Trusted Partners:
Worldwide Market Reports, Coherent MI, Stratagem Market Insights
Get
Recent News:
https://www.coherentmarketinsights.com/news
About Us:
Coherent Market Insights leads into
data and analytics, audience measurement, consumer behaviors, and market trend
analysis. From shorter dispatch to in-depth insights, CMI has exceled in
offering research, analytics, and consumer-focused shifts for nearly a decade.
With cutting-edge syndicated tools and custom-made research services, we
empower businesses to move in the direction of growth. We are multifunctional
in our work scope and have 450+ seasoned consultants, analysts, and researchers
across 26+ industries spread out in 32+ countries.
Contact Us:
Mr. Shah
Coherent Market Insights
533 Airport Boulevard,
Suite 400, Burlingame,
CA 94010, United States
US: + 12524771362
UK: +442039578553
AUS: +61-2-4786-0457
India: +91-848-285-0837

