Global Generic Sterile Injectables Market is estimated to be valued at USD 46.43 Bn in 2025 and is expected USD 93.39 Bn by 2032, reflecting a CAGR of 10.5% from 2025 to 2032. Generic sterile injectables serve as cost-effective and bioequivalent alternatives to branded injectables, produced under stringent regulatory standards such as those set by the U.S. FDA. Rising global incidences of chronic and non-communicable diseases are fueling market demand, with the World Health Organization (2023) reporting that NCDs are responsible for 74% of deaths worldwide.
Request Sample Report: https://www.coherentmarketinsights.com/insight/request-sample/519
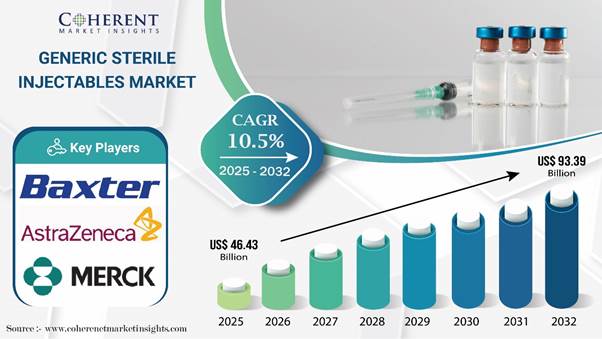
Global Generic Sterile Injectables Market Key Takeaways
Demand is anticipated to remain high for monoclonal antibodies, with the target segment accounting for 22.2% of market share in 2025.
Based on therapeutic application, cancer is slated to account for a prominent generic sterile injectables market share by 2025.
North America is set to account for nearly two-fifths of the global generic sterile injectables industry share by 2025.
Asia Pacific market is poised to emerge as a hotbed for generic sterile injectable manufacturers during the forecast period.
Rising Demand for Cost-Effective Medicines Fueling Market Growth
Coherent Market Insights’ latest generic sterile injectables market analysis outlines major factors driving the industry’s growth. Increasing demand for cost-effective medicines is one such prominent growth driver.
One of the most attractive benefits of generic sterile injectables is their cost-effectiveness. These drugs are priced lower than their branded counterparts. This makes them more affordable for patients as well as reduces healthcare costs, leading to their higher demand.
Read Also: Generic Drugs Market Size, Share, Trends & Opportunities for 2025-2032
Regulatory Hurdles Limiting Generic Sterile Injectables Market Growth
The global generic sterile injectables market outlook appears promising, considering the rising demand for cost-effective medicines and high prevalence of chronic diseases. However, regulatory challenges might limit market growth to some extent during the forecast period.
Sterile injectables need very strict standards like aseptic processing, sterility checks, and compliance with cGMP rules from the FDA, EMA, and others. These processes are costly and time-consuming, and even small issues such as contamination or sterilization failures can lead to recalls or penalties, which limit market growth.
Rising Prevalence of Chronic Diseases Unlocking Growth Prospects
The global incidence of chronic conditions such as diabetes, cancer, and hypertension is increasing rapidly. This rise is expected to drive demand for generic sterile injectables, which are often essential for long-term disease management and cost-effective treatment. So, the market for generic sterile injectables is set to grow strongly, creating good opportunities for manufacturers and stakeholders.
Immediate Delivery Available | Buy This Premium Research Report: https://www.coherentmarketinsights.com/insight/buy-now/519
Emerging Generic Sterile Injectables Market Trends
Increasing patent expirations are boosting growth of the generic sterile injectables market. When patents for many branded injectables expire, it creates room for generic versions. This lets pharmaceutical companies make cheaper alternatives of important medicines.
Rising adoption of biosimilars is expected to boost market growth in the coming years. Biosimilar injectables are being increasingly developed to offer cost-effective alternatives, especially in oncology and immunology.
Innovations in drug delivery systems are enhancing the convenience, safety, and efficacy of generic sterile injectables. Companies are developing innovative solutions like prefilled syringes, microneedle systems, and auto-injectors, many of which are designed for self-administration and user-friendly handling.
Growing trend of outsourcing drug development processes is supporting market expansion. Industry players are shifting toward contract development and manufacturing organizations (CDMOs) for numerous reasons.
Many governments are pushing generic adoption to reduce healthcare spending. This will likely play a key role in fostering growth of the generic sterile injectables industry during the forthcoming period.
Artificial intelligence is making its way into the generic sterile injectables industry. It is being used by companies to streamline drug discovery, optimize manufacturing processes, and enhance quality control. For instance, in 2024, Sanofi opened its EVolutive Facilities (EVF), state-of-the-art, fully digitalized manufacturing plants in France and Singapore, specifically designed for the production of biologics and sterile injectables.
Also Read: Injectable Drugs Market Analysis & Forecast for 2025-2032
Analyst’s View
“The global generic sterile injectables industry is set to witness strong growth, owing to rising demand for cost-effective drugs, increasing patent expirations of branded injectables, growing prevalence of chronic and infectious diseases, and expanding biologics and biosimilars pipeline,” said a senior CMI analyst.
Current Events and Their Impact on the Generic Sterile Injectables Market
|
Event |
Description and Impact |
|
Global Pharmaceutical Supply Chain Disruptions |
|
|
Healthcare Infrastructure Expansion in Emerging Markets |
|
|
Technological Disruption in Drug Delivery Systems |
|
Competitor Insights Key companies in generic sterile
injectables market report: - AstraZeneca plc - Baxter International Inc. - Merck & Co., Inc. - Novartis International AG - Teva Pharmaceuticals - Pfizer Inc. - Hikma Pharmaceuticals - Dr. Reddy’s Laboratory - Fresenius Kabi - Sun Pharmaceutical
Industries Ltd. - Mylan N.V. Key Developments ·
In
July 2025, Cronus Pharma LLC launched Butorphic (Butorphanol Tartrate) sterile
injectable solution in the U.S. This launch expands the company’s robust
sedative and anesthesia product portfolio. ·
In
August 2024, Fresenius Kabi launched new generic version of Cetrorelix Acetate for
injection. This medicine is used in reproductive health treatments. ·
In
March 2024, Ascendia Pharmaceutical Solutions opened a new sterile cGMP facility to
increase its production of injectable medicines. Request For Customization: https://www.coherentmarketinsights.com/insight/request-customization/519 Market
Segmentation Also Read: Small Molecule Injectable
Drugs Market Outlook for 2025-2032 Our Trusted Partners: Worldwide Market Reports, Coherent MI, Stratagem Market Insights Get Recent News: https://www.coherentmarketinsights.com/news About Us: Coherent
Market Insights leads into data and analytics, audience measurement, consumer
behaviors, and market trend analysis. From shorter dispatch to in-depth insights,
CMI has exceled in offering research, analytics, and consumer-focused shifts
for nearly a decade. With cutting-edge syndicated tools and custom-made
research services, we empower businesses to move in the direction of growth. We
are multifunctional in our work scope and have 450+ seasoned consultants,
analysts, and researchers across 26+ industries spread out in 32+ countries. Contact
Us: Mr. Shah Coherent
Market Insights Pvt. Ltd, U.S.: +
12524771362 U.K.:
+442039578553 AUS: +61-8-7924-7805 INDIA:
+91-848-285-0837 ✉ Email: sales@coherentmarketinsights.com

