According to Precedence Research, the global clinical trials market size is valued at USD 87.42 billion in 2025 and is estimated to grow from USD 91.66 billion in 2026 to nearly USD 149.58 billion by 2034, with a CAGR of 5.97% from 2025 to 2034. This growth is fueled by the rapid adoption of AI-driven trial designs, rising investments in precision medicine, and the expansion of next-generation therapies such as cell and gene treatments.
Increasing demand for adaptive and decentralized clinical trial models, along with strong activity in oncology and CNS research, continues to reshape the global research ecosystem. North America remains the dominant hub, while Asia-Pacific emerges as the fastest-growing region due to large patient pools and improving regulatory frameworks.

The Complete Study is Now Available for Immediate Access | Download the Sample
Pages of this Report@ https://www.precedenceresearch.com/sample/1185
Clinical Trials Market Key Insights at a Glance
🔹 North America remained the undisputed leader in 2024, commanding 59.31% of the global market thanks to its strong R&D ecosystem and robust regulatory infrastructure.
🔹 The Asia Pacific region is emerging as the fastest-growing hub, projected to expand at an impressive 7.16% CAGR, driven by large patient populations and rising investments in healthcare research.
🔹 From a study-design perspective, interventional trials dominated the field with 70.50% market share in 2024 due to their critical role in evaluating new therapies.
🔹 Meanwhile, expanded access programs are gaining traction, expected to grow at a strong 8.20% CAGR, reflecting rising demand for investigational therapies beyond traditional trial settings.
🔹 In terms of therapeutic focus, oncology remained the largest segment with 31.70% share, underscoring the global push toward innovative cancer treatments.
🔹 The CNS conditions segment is accelerating rapidly, projected to record 7.60% CAGR, driven by increasing prevalence of neurological disorders and advancements in neurotherapeutics.
🔹 Among service types, laboratory services led the market with 24.60% share in 2024, emphasizing the central role of diagnostics and biomarker testing in modern trials.
🔹 Decentralized clinical trial (DCT) services continue to reshape patient engagement and trial efficiency, expected to expand at a robust 9.40% CAGR over the forecast period.
➡️ Become a valued research partner with us ☎ https://www.precedenceresearch.com/schedule-meeting
Are Clinical Trials Entering the Most Transformative Decade Yet?
Clinical trials are undergoing a profound metamorphosis as science, technology, and global collaborations converge to redefine how new therapies reach patients. Once confirmed to rigid, site-centric designs, today’s trials increasingly embrace decentralised models, real-world evidence integration, and digital monitoring tools that enhance both precision and patient inclusivity. Sponsors are shifting from linear, slow-moving protocols to adaptive frameworks that allow mid-course corrections based on emerging data, significantly accelerating time-to-insight.
At the same time, rising biological complexity gene therapies, cell therapies, and rare-disease interventions demand highly specialised trial infrastructures and expert-driven operational ecosystems. Regulatory agencies worldwide are encouraging innovation while simultaneously tightening data integrity and safety expectations, pushing stakeholders toward more robust, tech-enabled quality systems. Together, these forces position the clinical trials market as an arena of unprecedented innovation, operational agility, and scientific ambition.
✚ Turn AI disruption into Opportunity. Click to Get the Insights Shaping Tomorrow.
What Factors are Fueling the Surge of Investment in Clinical Trials Today?
🔸 Investment in clinical trials is accelerating as breakthrough therapies especially in oncology, rare diseases, and cell and gene therapy demand sophisticated, large-scale validation before market entry.
🔸 The shift toward personalized medicine is compelling sponsors to run more targeted, biomarker driven studies, which require advanced analytics, specialised sites, and higher funding.
🔸 At the same time, digital transformation from remote patient monitoring to AI-powered protocol optimization is reducing operational friction and attracting investors seeking efficiency-driven returns.
🔸 Global health challenges and unmet patient needs are prompting governments, venture capitalists, and pharma companies to prioritise research pipelines with faster development cycles.
🔸 Rising regulatory support for adaptive trials and decentralised models is further incentivizing capital deployment by lowering long-term risk. Together, these forces are catalyzing a robust and future-forward investment landscape in the clinical trials sector.
What Barriers still Hinder the Adoption of Morden Clinical Trials?
The clinical trials landscape, despite its rapid evolution, continues to face several entrenched berries that slow the adoption of new methodologies and technologies. One major hurdle is the regulatory complexity, where varying global guidelines, prolonged approval cycles, and stringent compliance burdens often discourage innovation and delay trial initiation.
Patient recruitment and retention remain chronic challenges, as reaching diverse populations, maintaining engagement, and addressing logistical constraints limit participation. Additionally, high operational costs, ranging from sophisticated equipment to decentralized trial infrastructure, pose financial obstacles, especially for smaller sponsors.
📥 Dive into the Complete Report ➡️ https://www.precedenceresearch.com/clinical-trials-market
Clinical Trials Market Report Coverage and Framework
|
Report Highlights |
Details |
|
Market Growth Rate (2025-2034) |
CAGR of 5.97% |
|
Market Size in 2025 |
USD 87.42 Billion |
|
Market Size in 2026 |
USD 91.66 Billion |
|
Market Size by 2034 |
USD 149.58 Billion |
|
Dominating Region in 2024 |
North America (59.31% Market Share) |
|
Fastest Growing Region (2025-2034) |
Asia Pacific (CAGR of 7.16%) |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2034 |
|
Segments Covered |
Study Design, Indication, Service Type, and Region |
|
Companies Mentioned |
Parexel, IQVIA, Charles River Laboratory, Omnicare, Kendle, Chiltern, Pharmaceutical Product Development, LLC |
|
Key Study Design Insights |
Interventional studies: 70.50% share (2024); Expanded access: CAGR 8.20% forecast |
|
Key Indication Insights |
Oncology: 31.70% market share (2024); CNS conditions: CAGR 7.60% forecast |
|
Key Service Type Insights |
Laboratory services: 24.60% share (2024); Decentralized clinical trial services: CAGR 9.40% forecast |
|
Growth Drivers |
Rising prevalence of chronic disorders, increasing clinical trials in developing regions, biologics demand, personalized medicine adoption |
|
Industry Trends |
Outsourcing of R&D activities, expansion of CRO services beyond preclinical and clinical trials into project management, safety audits, and bio-statistical analysis |
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Case Study: Decentralized Clinical Trials (DCT) in Oncology – Transforming Patient Engagement and Trial Efficiency
Background:
Traditional clinical trials often face challenges like slow patient
recruitment, high operational costs, and limited geographic reach. Oncology
trials, in particular, require large, diverse patient populations and frequent
monitoring, making conventional site-centric trials resource-intensive and
time-consuming.

Objective:
To evaluate the impact of decentralized clinical trials (DCTs) on recruitment
efficiency, patient retention, and operational cost in Phase II/III oncology
studies.
Implementation:
A leading Contract Research Organization (CRO)
partnered with multiple biopharmaceutical companies to
conduct decentralized oncology trials across North America
and Europe. Key features included:
- Telemedicine Visits: Routine follow-ups conducted remotely via secure video conferencing.
- Home Health Services: Blood draws, vitals monitoring, and sample collection performed at patients’ homes.
- Digital Data Capture: Electronic patient-reported outcomes (ePROs) and wearable devices integrated for real-time monitoring.
- AI-driven Patient Matching: Machine learning algorithms used to identify eligible patients faster and optimize site selection.
Key Findings:
- Faster Recruitment: Enrollment rates increased by 35%, as patients from remote areas could participate without relocating.
- Higher Retention: Drop-out rates decreased by 20% due to reduced travel burden and improved convenience.
- Operational Cost Savings: Trial costs were reduced by approximately 15% due to fewer site visits, optimized logistics, and streamlined monitoring.
- Data Quality: Integration of real-time monitoring and digital endpoints enhanced accuracy and timeliness of data submission to regulators.
- Regulatory Approval Support: Sponsors reported smoother interactions with regulatory authorities, as DCT models demonstrated patient safety and protocol adherence.
Strategic Implications:
- For Sponsors: DCT adoption allows faster go-to-market timelines for oncology drugs and reduces trial bottlenecks in recruitment and retention.
- For Investors: Investment in DCT infrastructure, AI-driven trial design, and digital health solutions presents high ROI potential.
- For Emerging Markets: DCT frameworks can accelerate clinical research in Asia-Pacific by leveraging large patient pools without requiring extensive physical site networks.
Conclusion:
This case study demonstrates how decentralized clinical trials, combined with
digital tools and AI, are reshaping the clinical trials ecosystem. Sponsors,
CROs, and investors who embrace these innovations are better positioned to
optimize costs, accelerate development timelines, and improve patient-centric
outcomes.
Don’t Miss Out! | Grab Your Discounted Report Before Prices Go Up! 👉 https://www.precedenceresearch.com/checkout/1185
Clinical Trials Market Regional Outlook
Why Does North America Lead the Clinical Trials Market?
North America commands the clinical trials landscape because it hosts one of the world’s most sophisticated and mature research ecosystems. The region benefits from robust funding, both public and private, which enables extensive therapeutic innovation and large-scale trial execution. Its well-established regulatory framework, though stringent, offers clarity, structure, and predictable approval pathways that attract global sponsors.
What is the U.S. Clinical Trials Market Size?
According to Precedence Research, the U.S. clinical trials market size is projected to reach USD 82.79 billion by 2034, increasing from USD 47.18 billion in 2025. The market is growing at a strong CAGR of 6.40% from 2025 to 2034.
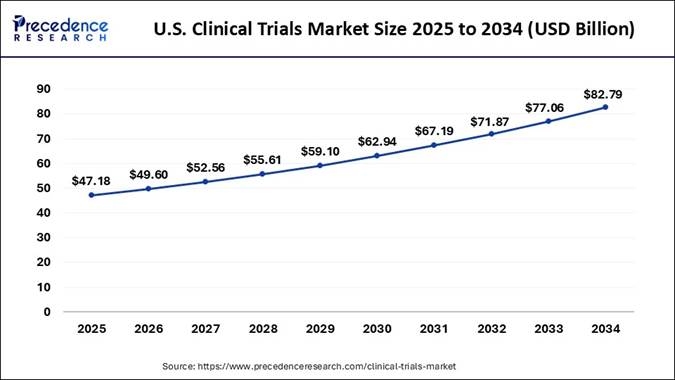
U.S. Clinical Trials Market: Booming Momentum Till 2030
The U.S. clinical-trials market is experiencing strong and sustained growth thanks to a confluence of favorable factors. Broad adoption of novel therapies (especially in oncology, rare diseases, and advanced biologics), rising demand for personalized medicine, and a robust biopharmaceutical R&D ecosystem are fueling a surge in trial activity.
At the same time, technological innovations, including use of AI, big-data analytics, decentralized trials (telemedicine, remote monitoring), and cloud-based clinical-trial management systems are markedly improving trial design, patient recruitment, data collection, and overall speed and efficiency.
Note: This report is readily available for
immediate delivery. We can review it with you in a meeting to ensure data
reliability and quality for decision-making.
📥 Download
Sample Pages for Informed Decision-Making 👉 https://www.precedenceresearch.com/sample/2480
U.S. Clinical Trials Market Leading Companies
➢ Parexel International Corp.
➢ Charles River Laboratory
➢ PRA Health Sciences
➢ Wuxi AppTec
➢ Eli Lilly and Company
➢ Novo Nordisk A/S
➢ Clinipace
➢ Omnicare
➢ Kendle
➢ Chiltern

Why is Asia Pacific the Fastest Growing Clinical Trials Market?
Asia-Pacific is witnessing the fastest growth in clinical trials due to its unique blend of demographic, economic, and scientific advantages. The region offers large, treatment-naïve, and diverse patient populations, enabling faster recruitment one of the biggest bottlenecks in global trials. Rapid improvements in healthcare infrastructure, research capabilities, and digital adoption are dramatically enhancing the region’s readiness for sophisticated clinical studies.
India: Government Support and Regulatory Facilitation Fuel Market Growth
In India, government-led initiatives and regulatory reforms have significantly improved the clinical-trials environment, creating growing opportunities for domestic and global trials. The introduction of updated regulations under the New Drugs and Clinical Trials Rules (NDCT) of 2019 streamlined approval processes, reducing waiting times for trial permissions which encourages faster initiation of studies.
🔸 Key institutions under the Indian Council of Medical Research (ICMR) have been designated as Clinical Trial Units (CTUs), enabling India to conduct early-phase (Phase I) trials for indigenous drugs, including cancer therapies.
Moreover, rising R&D investments, expansion of pharmaceutical and biotech sectors, a growing skilled workforce, and increasing disease burden (e.g., chronic diseases, growing demand for advanced treatments) are collectively driving growth in clinical-trial activity. The combined effect of regulatory facilitation and rising demand for innovative treatments is helping India emerge as an increasingly important hub for clinical research.
Clinical Trials Market Segmentation Insights:
Study Design Insights
Why Are Interventional Studies Dominating the Clinical Trials Markets?
Interventional studies continue to dominate the clinical trials landscape because they are the cornerstone of testing new therapies and assessing efficacy under controlled conditions. These studies allow sponsors to directly measure outcomes by actively administering treatments to participants, providing critical data for regulatory approvals. Their structured methodology ensures reliability, reproducibility, and robust safety monitoring, making them the preferred approach for most therapeutic areas. As a result, interventional studies remain the backbone of clinical research, driving the majority of ongoing trials globally.
Expanded access programs are rapidly gaining traction as they allow patients to receive investigational treatments outside of traditional trials. This approach is particularly valuable for life-threatening or rare conditions where conventional study participation may be limited. By offering earlier access to promising therapies, these programs enhance patient engagement and build real-world evidence for emerging treatments. Consequently, expanded access represents a dynamic growth segment, reflecting both patient-centricity and evolving regulatory support.
Indication Insights
Why Does Oncology Dominate the Clinical Trials Market?
Oncology remains the leading indication in clinical trials due to the urgent global need for innovative cancer therapies. High prevalence, complex disease biology, and the continuous development of targeted and immunotherapies drive substantial research investments. Oncology trials often involve large patient populations and multiple study sites, generating significant volumes of clinical data. This consistent focus ensures that oncology maintains its dominant share across therapeutic areas in the clinical trials landscape.
Central nervous system (CNS) conditions are emerging as the fastest-growing segment due to rising prevalence of neurological disorders and mental health challenges worldwide. Advances in neuropharmacology, biomarker discovery, and imaging technologies are enabling more sophisticated trial designs. Increasing awareness, patient advocacy, and regulatory incentives are further accelerating clinical research in CNS therapeutics. These factors collectively contribute to the rapid growth and heightened focus on CNS-related trials.
Service Type Insights
Why Are Laboratory Services Dominating the Clinical Trials Market?
Laboratory services dominate the clinical trials market because they provide essential support for sample collection, biomarker analysis, and high-precision diagnostics. Accurate laboratory testing underpins the integrity of clinical data, ensuring safety and efficacy assessments are reliable. Sponsors heavily rely on centralized labs for standardized protocols, quality assurance, and regulatory compliance. This indispensable role keeps laboratory services at the forefront of clinical trial operations globally.
Decentralized clinical trials are growing rapidly as they leverage digital technologies to bring trials closer to patients, reducing the need for frequent site visits. Telemedicine, remote monitoring, and home-based assessments enhance patient convenience, recruitment, and retention rates. These trials also generate more real-world, patient-centric data, which is increasingly valued by regulators and sponsors alike. As clinical research evolves toward flexibility and accessibility, decentralized trials have emerged as the fastest-growing service segment.
✚ Related Topics You May Find Useful:
➡️ AI in Clinical Trials Market: Explore how artificial intelligence is optimizing trial design, data analysis, and patient monitoring.
➡️ Clinical Trials Site Management Organizations Market: Understand the growing role of site management organizations in streamlining trial operations.
➡️ Virtual Clinical Trials Market: See how decentralized trial models are enhancing patient access and engagement.
➡️ Clinical Trial Supply and Logistics Market: Analyze efficient supply chain solutions supporting global clinical studies.
➡️ Mental Health Clinical Trials Market: Track the rising demand for innovative therapies in mental health research.
➡️ Cell and Gene Therapy Clinical Trials Market: Gain insight into cutting-edge trials shaping personalized medicine.
➡️ RNA Therapy Clinical Trials Market: Explore how RNA-based therapies are transforming treatment development.
➡️ Oncology Clinical Trials Market: Understand evolving trends in cancer research and therapeutic testing.
➡️ Clinical Trial Biorepository and Archiving Solutions Market: Discover solutions ensuring secure sample storage and data integrity.
➡️ Clinical Trial Patient Recruitment Services Market: Analyze strategies improving enrollment and retention in clinical studies.
➡️ AI-Based Clinical Trials Solution Provider Market: Explore providers leveraging AI to enhance trial efficiency and outcomes.
➡️ Clinical Trial Supplies Market: Track innovations in providing essential materials for global trials.
➡️ Biopharma Clinical Trials Market: Gain insight into how biopharmaceutical research is driving new therapy development.
What is Going Around the Globe in Clinical Trials Landscape?
The clinical trials market is experiencing a remarkable transformation globally, driven by technological innovation, patient-centric approaches, and an ever-expanding therapeutic horizon. Across regions, sponsors are increasingly adopting decentralized and hybrid trial models, enabling participants to engage from home and improving recruitment efficiency. Advanced digital tools, AI, and real-world data integration are redefining how trials are designed, monitored, and analyzed, creating faster, more accurate, and cost-effective outcomes.
Oncology remains a key focus, but there is growing attention toward rare diseases, CNS disorders, and gene therapies, reflecting both unmet medical needs and regulatory encouragement. Emerging markets in Asia-Pacific and Latin America are witnessing a surge in trial activity due to large, treatment-naïve patient populations and improving healthcare infrastructure, while North America and Europe continue to lead in investment, advanced technology adoption, and regulatory expertise.
Recent Developments in Clinical Trials Market:
🔸 In January 2025, a new antibody-drug conjugate (ADC) known as IPH4502 entered clinical evaluation with the first patient dosed in a Phase 1 trial. The therapy targets Nectin-4 and is engineered with an advanced topoisomerase inhibitor payload, positioning it as a potential treatment for a broad range of solid tumors. This marks a significant step forward in expanding next-generation ADC options within early-stage clinical pipelines.
🔸 In February 2025, the first patient was enrolled in a Phase III clinical study evaluating Ivonescimab combined with chemotherapy for first-line treatment of advanced triple-negative breast cancer. Ivonescimab, a bispecific antibody that targets both PD-1 and VEGF, represents a newer immunotherapy approach designed to enhance immune activation while inhibiting tumor angiogenesis, signaling continued momentum in innovative biologic therapies.
Top Companies in the Clinical Trials Market
➢ Parexel
➢ IQVIA
➢ Omnicare
➢ Kendle
➢ Chiltern
➢ Pharmaceutical Product Development, LLC
Clinical Trial Market Segmentations:
By Study Design
🔹 Interventional
🔹 Observational
🔹 Expanded Access
By Indication
🔹 Autoimmune/Inflammation
→ Rheumatoid arthritis
→ Multiple Sclerosis
→ Osteoarthritis
→ Irritable Bowel Syndrome (IBS)
→ Others
🔹 Pain Management
→ Chronic Pain
→ Acute Pain
🔹 Oncology
→ Blood Cancer
→ Solid Tumors
→ Other
🔹 CNS Condition
→ Epilepsy
→ Parkinson's Disease (PD)
→ Huntington's Disease
→ Stroke
→ Traumatic Brain Injury (TBI)
→ Amyotrophic Lateral Sclerosis (ALS)
→ Muscle Regeneration
→ Others
🔹 Diabetes
🔹 Obesity
🔹 Cardiovascular
🔹 Others
By Service Type
🔹 Laboratory Services
🔹 Bioanalytical Testing Services
🔹 Decentralized Clinical Trial Services
🔹 Patient Recruitment
🔹 Site Identification
🔹 Analytical Testing Services
🔹 Clinical Trial Supply & Logistic Services
🔹 Other Services
By Region
🔹 North America
🔹 Europe
🔹 Asia-pacific
🔹 Latin America
🔹 Middle and East Africa
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or Asia Pacific.
Don’t Miss Out! | Grab Your Discounted Report Before Prices Go Up! 👉 https://www.precedenceresearch.com/checkout/1185
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344
Stay Ahead with Precedence Research Subscriptions
Unlock exclusive access to powerful market intelligence, real-time data, and forward-looking insights, tailored to your business. From trend tracking to competitive analysis, our subscription plans keep you informed, agile, and ahead of the curve.
Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription
About Us
Precedence Research is a global market intelligence and consulting powerhouse, dedicated to unlocking deep strategic insights that drive innovation and transformation. With a laser focus on the dynamic world of life sciences, we specialize in decoding the complexities of cell and gene therapy, drug development, and oncology markets, helping our clients stay ahead in some of the most cutting-edge and high-stakes domains in healthcare. Our expertise spans across the biotech and pharmaceutical ecosystem, serving innovators, investors, and institutions that are redefining what’s possible in regenerative medicine, cancer care, precision therapeutics, and beyond.
Web: https://www.precedenceresearch.com
✚ Explore More Market Intelligence from Precedence Research:
➡️ Digital Therapeutics: How software-based interventions are restructuring chronic-disease management and clinical-grade behavioral therapy
➡️ Life Sciences Growth: Forces driving expansion across biotech, biopharma, and advanced therapeutic platforms
➡️ Viral Vector Gene Therapy Manufacturing: Manufacturing constraints, scalability limits, and innovations shaping next-generation gene-delivery systems
➡️ Wellness Transformation: How prevention-centric health models are shifting consumer behavior, product pipelines, and care delivery
➡️ Generative AI in Healthcare: How generative models are unlocking new diagnostics, clinical automation, and patient-care innovations
Our Trusted Data Partners:
Towards Healthcare | Nova One Advisor | Onco Quant | Statifacts
Get Recent News 👉 https://www.precedenceresearch.com/news
For Latest Update Follow Us:

