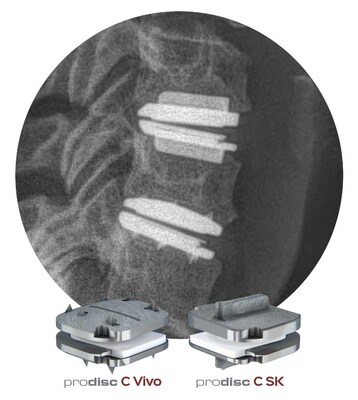
- The prodisc® C Vivo and prodisc® C SK are now indicated for reconstruction of a vertebral disc from C3-C7 following discectomy at one level or two contiguous levels.
- A landmark IDE Clinical Study successfully demonstrated the safety and clinical effectiveness of prodisc C Vivo and prodisc C SK, achieving the highest overall composite clinical success rate at two-levels as compared to any other approved cervical total disc replacement (TDR) device.1
- The prodisc C Vivo and prodisc C SK Match-the-Disc™ System has now become the first and only cervical TDR system with two different devices approved for both one- and two-level use.
- Centinel Spine's prodisc technology is now the only TDR solution in the U.S. approved for one- and two-level use in both the cervical and lumbar spine.
WEST CHESTER, Pa., Oct. 14, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced U.S. Food and Drug Administration (FDA) Premarket Approval (PMA) for 2-level indications for the prodiscC Vivo and prodisc C SK Cervical TDR devices. Since receiving FDA approval for 1-level indications in July 2022, nearly 20,000 prodiscC Vivo and prodisc C SK spinal levels have been implanted in the U.S. by over 1,100 surgeons.
"It's an exceptional day for cervical arthroplasty!" commented Armen Khachatryan MD, Orthopedic Spine Surgeon at The Disc Replacement Center in Salt Lake City, UT. "I am thrilled to witness the culmination of our efforts in the prodiscC Vivo and prodiscCSK clinical trial for two-level indications, resulting in FDA approval. I am particularly enthusiastic about integrating the distinct endplate configurations of the prodisc devices into my clinical practice. This approval expands the range of treatment options available to patients and underscores the continuous evolution and refinement of motion-preserving surgical techniques for cervical spine disorders," Dr. Khachatryan concluded.
A total of 480 subjects were enrolled in the IDE Clinical Study across 31 centers, and the PMA is based on the analysis of 433 subjects—which is the highest number of subjects used to support a PMA for any cervical TDR device on the market. The Clinical Study was the first of its kind to evaluate two investigational TDR devices and the first PMA based on an IDE Clinical Study that compares an investigational system to a PMA approved cervical TDR device.
According to Dr. Jad Khalil, Orthopedic Spine Surgeon with Michigan Orthopaedic Surgeons, Southfield, MI, "Cervical disc arthroplasty is a great alternative to traditional fusion surgery. Multiple clinical trials have shown excellent outcomes, and our research group was fortunate to be a part of this breakthrough clinical trial on the new generation of prodisc cervical devices for 2-level arthroplasty. What sets the prodiscC Vivo and prodiscC SK system apart is the ability to match the implant to the patient anatomy for a more tailored approach to the procedure. We are excited to see the release of the expanded indications for this system."
For study patients who reached the 24-month endpoint, the IDE Clinical Study results demonstrated:
- Patients treated with prodisc C Vivo and prodisc C SK had statistically non-inferior (i.e. equivalent) outcomes versus patients treated with the control cervical TDR device.2
- Patients treated with prodisc C Vivo and prodisc C SK achieved an overall composite clinical success rate of 87.1% (vs. 83.7% control cervical TDR)2, which is the highest overall composite clinical success rate at two levels compared to any other approved cervical TDR device.1
- 93.9% of prodisc C Vivo and prodisc C SK patients achieved a meaningful (15 point) improvement in Neck Disability Index (NDI) vs. 90.1% for the control cervical TDR.2
- 96.9% of prodisc C Vivo and prodisc C SK patients had no secondary surgical interventions (revision, removal, re-operation, supplemental fixation) at the index level(s) vs. 95.7% for the control cervical TDR.2
Centinel Spine's CEO, Steve Murray, summed up, "This is a historic milestone for Centinel Spine and further advances treatment options for spine surgeons and their patients. The IDE trial supporting this FDA approval is a landmark study using another FDA-approved disc device as a control and including two different investigational devices that could be used for the same patient. This 2-level approval advances the concept of uniquely matching the disc to each level of the patient's cervical spine and is a major step forward in total disc replacement."
The prodisc C Vivo has been in clinical use internationally since 2009 and is currently one of the most frequently implanted TDR devices in the world. The device offers keel-less insertion and combines a unique anatomically-designed superior endplate with lateral spikes to optimize fit and provide immediate fixation. The prodisc C SK device features a flat endplate designed for optimized implant positioning that allows surgeons to address individual patient anatomy—with a low-profile central keel that offers immediate fixation and enables a streamlined keel preparation technique. Both devices incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of every prodisc device after over 35 years and 275,000 implantations, worldwide.3
1 The Study Success Rate reported is based on the Overall Success rate of the Primary Analysis Population in the SSED for each cervical total disc replacement device with two level indications.
2 For detailed information regarding the IDE study, refer to the Summary of Safety and Effectiveness Data at 2level.centinelspine.com.
3 Data on file at Centinel Spine
About Centinel Spine, LLC
Centinel Spine®, LLC is the leading global medical device company exclusively focused on addressing cervical and lumbar spinal disease with prodisc®, the most complete total disc replacement (TDR) technology platform in the world.
The Company's prodisc technology is the most studied and clinically-proven TDR system across the globe, validated by over 540 published papers and more than 275,000 implantations. Centinel Spine's prodisc is the only TDR technology with multiple motion-preserving anatomic solutions, allowing the surgeon to Match-the-Disc™ to each patient's anatomy for both cervical and lumbar total disc replacement.
For more information, please visit the company's website at www.CentinelSpine.com or contact:
Varun Gandhi
Chief Financial Officer
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: v.gandhi@centinelspine.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/centinel-spine-receives-two-level-fda-approval-for-prodisc-c-vivo-and-prodisc-c-sk-match-the-disc-cervical-total-disc-replacement-devices-302582526.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/centinel-spine-receives-two-level-fda-approval-for-prodisc-c-vivo-and-prodisc-c-sk-match-the-disc-cervical-total-disc-replacement-devices-302582526.html
SOURCE Centinel Spine, LLC