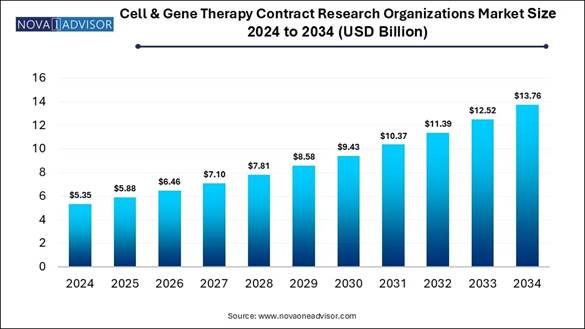
According to Nova One Advisor, the global cell & gene therapy contract research organizations market size is calculated at USD 5.35 billion in 2024, grows to USD 5.88 billion in 2025, and is projected to reach around USD 13.76 billion by 2034, growing at a CAGR of 9.91% from 2025 to 2034.
The cell & gene therapy contract research organizations market is expanding due to CRO offering numerous advantages for cell and gene therapy, such as speed, expertise, and flexibility. CRO is that it authorizes sponsors to make informed decisions, keep trial quality, use resources effectively, and reduce interruptions. It offers auditable financials and improves resource allocation to lower operational expenses of cell and gene therapies.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/9185
Cell & Gene Therapy Contract Research Organizations Market Highlights:
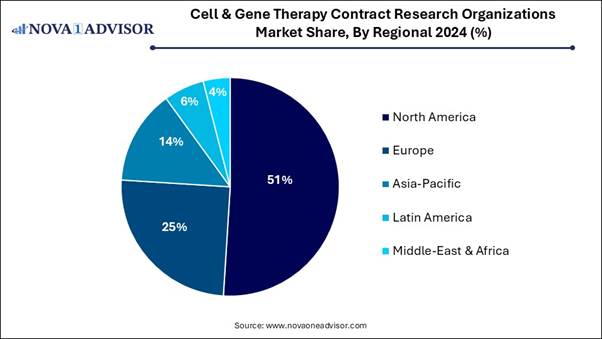
• North America dominated the global bone regeneration material market in 2024.
• Asia Pacific is expected to grow at the fastest CAGR over the forecast period.
• By type, the clinical segment dominated the market with the largest share in 2024.
• By type, the preclinical segment is expected to show the fastest growth over the forecast period.
• By service, the clinical monitoring segment accounted for the highest market share in 2024.
• By service, the regulatory strategy segment is expected to expand rapidly during the predicted timeframe.
• By indication, the oncology segment held the largest market share in 2024.
• By indication, the CNS disorders segment is expected to register the fastest growth during the forecast period.
• By modality, the cell-based therapies segment captured the largest market share in 2024.
• By modality, the gene therapies segment is expected to show the fastest growth during the forecast period.
Market Overview and Industry Potential
The cell and gene therapy contract research organizations market is expanding due to CROs bringing valuable resources and abilities to the cell and gene therapies, which accelerates timelines and efficient processes through government expertise and access to advanced technologies. CROs have a deep understanding of the governing landscape and can navigate the complex maze of regulations and guidelines that govern cell and gene therapy research. They have dedicated teams of specialists who stay up to date with the modern regulatory changes and ensure that the study remains compliant throughout its period. This level of government expertise helps avoid costly delays and ensures that research progresses efficiently.
⬥︎ For Instance, In September 2024, Cryoport, Inc., a global leader in supply chain solutions for the life sciences industry, and SK pharmteco, a worldwide contract development and manufacturing organization, announced a strategic collaboration to provide fully integrated logistics and manufacturing services to biotechnology and pharmaceutical companies.
Continuous increasing interest in cell and gene therapy outsourcing provides access to a specialised organization’s internal resources, including its equipment and in-house specialists. Working with a proficient CRO, customers usually scale up faster with their services. CRO continuously examine and progress their portfolios and what they can offer to their novel cell and gene therapy customers.
CRO to handle entirely aspects of a clinical trial or advancement program, fundamentally end-to-end. The CRO is responsible for the complete study operations, and the sponsor’s contribution is primarily oversight and decision-making.
What are the Latest Trends of Cell & Gene Therapy Contract Research Organizations Market?
⬥︎ In July 2025, the Centers for Medicare & Medicaid Services (CMS) announced that 33 states, plus the District of Columbia and Puerto Rico, will participate in the Cell and Gene Therapy (CGT) Access Model, a bold novel strategy to delivering advanced treatments for people on Medicaid living with sickle cell disease. Participating states represent approximately 84% of Medicaid beneficiaries with the condition, significantly expanding access to transformative care.
⬥︎ In April 2025, Charles River Laboratories International, Inc. announced the initial cohort of its Charles River Incubator Program (CIP). The program offers early-stage biotechnology pioneers access to extensive scientific and commercial expertise and a wide ecosystem of discovery, development, and manufacturing capabilities to expedite the development of technologies and life-changing therapies for patients in need.
Increasing Demand for Personalized Cell Therapies: Market’s Largest Potential
Growing demand for personalize cell therapies as it provides major advantages, ranging from enhancing diagnostic precision to recognizing the ideal treatment option for a patient based on their characteristics, to targeted therapy that rises the likelihood of effective treatments, lowers adverse effects, enables for better disease prevention, and most significantly, increases patient engagement, lowers medical care expenses, and promotes research and revolution, so these create strong demand of scalable, flexible and rapid advancement platforms, CROs are particularly good at providing.
⬥︎ For Instance, In February 2024, AstraZeneca expands its US manufacturing footprint to accelerate ambitions in next-generation cell therapy discovery and development. AstraZeneca is investing $300 million in an advanced facility in Rockville, MD, to launch its life-saving cell therapy platforms in the US for critical cancer trials and future commercial supply.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/9185
Report Scope of Cell & Gene Therapy
Contract Research Organizations Market
|
Report Coverage |
Details |
|
Market Size in 2025 |
USD 5.88 Billion |
|
Market Size by 2034 |
USD 13.76 Billion |
|
Growth Rate From 2025 to 2034 |
CAGR of 9.91% |
|
Base Year |
2024 |
|
Forecast Period |
2025-2034 |
|
Segments Covered |
Type, Service, Indication, Modality, Region |
|
Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Key Companies Profiled |
Altasciences, Allucent, ICON plc, Labcorp, Linical, Medpace, Thermo Fisher Scientific Inc., Precision Medicine Group, LLC., QPS Holdings, Syneos Health |
Cell & Gene Therapy Contract Research Organizations Market Segmentation Analysis:
By Type Analysis:
The clinical segment dominates in the cell and gene therapy contract research organization market, as cell and gene therapy in clinical trials involves a stretch of DNA to prevent or manage a genetic condition. It's presently applied for treating various types of blood cancers and a small number of rare genetic conditions. These trials lead to long-lasting or even one-time treatments, potentially avoiding the requirement for continuing medication.
On the other hand, the preclinical segment is expected to grow significantly during the forecast period, as CRO conducts preclinical research and offer data to support drug or device development in early stages. It conducts preclinical research on behalf of biopharmaceutical or life science organizations. CROs are often applied for preclinical studies in fields like cell and gene therapy, immunology, and oncology. This model removes the requirement for in-house lab space and personnel.
By Service Analysis:
The clinical monitoring segment dominated the market in 2024, as it ensures trial success with person-centric specialists, custom site funding, and operative excellence in all phases. Monitoring in clinical trials supports study sponsors to assess and prove study data, improve patient safety, and ensure protocol agreement, in real-time and from a centralized location, and with significantly lower requirements for in-person site monitoring visits.
On the other hand, the regulatory strategy segment is expected to grow at the fastest CAGR in the market during the forecast period, as an efficient regulatory strategy is a game changer for organizations, improving potential revenue, lowering product failure rates, and bringing much-needed therapies to patients. A clear regulatory strategy includes quickening timelines, cost incentives, and the chance to partner with the government to ensure a smooth advancement programme.
By Therapeutic Area Analysis:
The oncology segment dominated the market in 2024, as cancer is the most common disease in gene therapy clinical trials. Cancer gene therapy mainly focuses on removing the cancer cells, blocking tumor vascularization, and improving the immune response to cancer antigens. Many gene and cell therapy strategies are being discovered for the treatment of a diversity of acquired diseases.
On the other hand, the CNS disorders segment is expected to grow at the fastest CAGR in the market during the forecast period, as gene therapy is significant for central nervous system (CNS) disorders, opening up a compelling possible for the development of advanced therapies. It provides the potential of transformative and disease-modulating management opportunities.
By Modality Analysis:
The cell-based therapies segment dominated the market in 2024, as cell-based therapy, particularly stem cells, offers a new hope for patients suffering from irredeemable diseases, where treatment strategies focus on the treatment of the disease rather than treating it. It is a significant branch of regenerative medicine with the ultimate aim of improving the body's repair machinery through modulation, stimulation, and regulation of the endogenous stem cell population and replenishing the cell pool toward tissue regeneration and homeostasis.
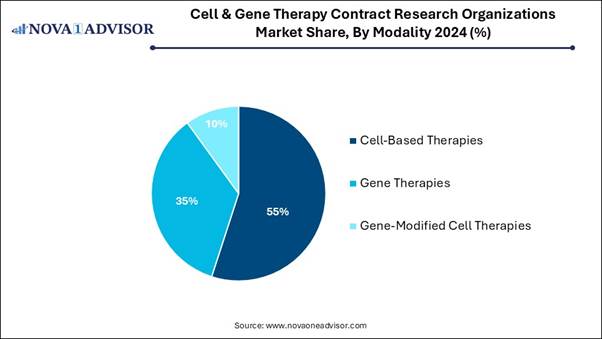
On the other hand, the gene therapies segment is expected to grow at the fastest CAGR in the market during the forecast period, as gene therapies are applied to avoid, manage, or cure some inherited disorders, like cystic fibrosis, alpha-1 antitrypsin deficiency, beta thalassemia, haemophilia, and sickle cell disease. They may also be used to manage and treat cancers or infections, including HIV. Gene therapy has huge potential to get rid of a patient’s symptoms for life. Gene therapy provides many people with an improved quality of life.
By Regional Insights
North America dominated the cell and gene therapy contract research organization market in 2024, due to it has strong research ecosystem as well as federal funds that have supported academic research, which in turn, has boosted private advancement, increasing discoveries in technology, medicine, and advanced cell and gene therapies, which drives the growth of the market. Growing investments in health research and development (R&D), supported by private funding and government grants, also contribute to the growth of the market.
⬥︎ For Instance, In October 2024, Minaris Regenerative Medicine, LLC and TFBS Bioscience Inc. are pleased to announce a strategic partnership in cell and gene therapy (CGT), mainly in North America. This collaboration is designed to improve their abilities to drive industry innovation by providing one-stop-shop solutions.
There were 4,321 contract research organizations in the U.S. businesses as of 2024, a rise of 2.1% from 2023, which adopt new technology of cell and gene therapies. U.S. leadership in novel drugs and medical devices is gaining regulatory approval. The country also ranks at the top in scientific Nobel prizes per capita, scientific impact in academia, and research and development expenses per capita, which drive the growth of the market.
Why Asia Pacific is the Fastest Growing in the Cell & Gene Therapy Contract Research Organizations Market?
Strong presence of leading CROs in Asia Pacific, such as Novotech, Charles River Laboratories, Labcorp (Covance), Medpace, and Caidya, drives the growth of the market. Increasing government support for rare diseases research and gene therapy approvals, increasing outsourcing, and revolution, which contribute to the growth of the market.
Cell & Gene Therapy Contract Research Organizations Market Companies:
• Allucent
• ICON plc
• Labcorp
• Linical
• Medpace
• Thermo Fisher Scientific Inc.
• Precision Medicine Group, LLC.
• QPS Holdings
What is Going Around the Globe?
⬥︎ In April 2025, AGC Biologics Launched a New Dedicated Cell and Gene Business Division. The new Cell and Gene Technologies Division will focus on elevating existing AGC Biologics capabilities and supporting developers in need of capacity, scientific capabilities, and technically qualified cell and gene CDMO operators
⬥︎ In June 2024, Cryoport, Inc., a global leader in supply chain solutions for the life sciences, and Minaris Regenerative Medicine Co., Ltd., a global contract development and manufacturing organization for cell and gene therapies, announced a collaborative partnership to offer fully integrated logistics and manufacturing solution to biotechnology and pharmaceutical companies for regenerative medicine products to helps the advancement of cell and gene therapies.
You can place an order or ask any questions, please feel free to contact at sales@novaoneadvisor.com | +1 804 441 9344
Related Report
⬥︎ U.S. DNA Manufacturing Market - https://www.novaoneadvisor.com/report/us-dna-manufacturing-market
⬥︎ Cell Culture Market - https://www.novaoneadvisor.com/report/cell-culture-market
⬥︎ Cell & Gene Therapy Bioanalytical Testing Services Market - https://www.novaoneadvisor.com/report/cell-gene-therapy-bioanalytical-testing-services-market
⬥︎ U.S. Plasmid DNA Contract Manufacturing Market - https://www.novaoneadvisor.com/report/plasmid-dna-contract-manufacturing-market
⬥︎ Clinical Trials Market - https://www.novaoneadvisor.com/report/clinical-trials-market
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the cell & gene therapy contract research organizations market.
By Type
• Drug Discovery
o Target Validation
o Lead Identification
o Lead Optimization
• Preclinical
• Clinical
o Phase I
o Phase II
o Phase III
o Phase IV
By Service
• Project & Clinical Trial Management
• Regulatory Strategy
• Data Management & Medical Writing
• Clinical Monitoring
• Quality Management / GMP Compliance
• Biostatistics & Safety Monitoring
• Patient & Site Recruitment
• Technology Transfer
• Others
By Indication
• Oncology
• CNS Disorders
• Infectious Diseases
• Immunological Disorders
• Cardiovascular Diseases
• Respiratory Diseases
• Diabetes
• Ophthalmology
• Pain Management
• Others
By Modality
• Cell-Based Therapies
• Gene Therapies
• Gene-Modified Cell Therapies
By Regional
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East and Africa (MEA)
Immediate Delivery Available | Buy This Premium Research https://www.novaoneadvisor.com/report/checkout/9185
About-Us
Nova One Advisor is a global leader in market intelligence and strategic consulting, committed to delivering deep, data-driven insights that power innovation and transformation across industries. With a sharp focus on the evolving landscape of life sciences, we specialize in navigating the complexities of cell and gene therapy, drug development, and the oncology market, enabling our clients to lead in some of the most revolutionary and high-impact areas of healthcare.
Our expertise spans the entire biotech and pharmaceutical value chain, empowering startups, global enterprises, investors, and research institutions that are pioneering the next generation of therapies in regenerative medicine, oncology, and precision medicine.
Web: https://www.novaoneadvisor.com/
Contact Us
USA: +1 804 420 9370
Email: sales@novaoneadvisor.com
For Latest Update Follow Us: LinkedIn

