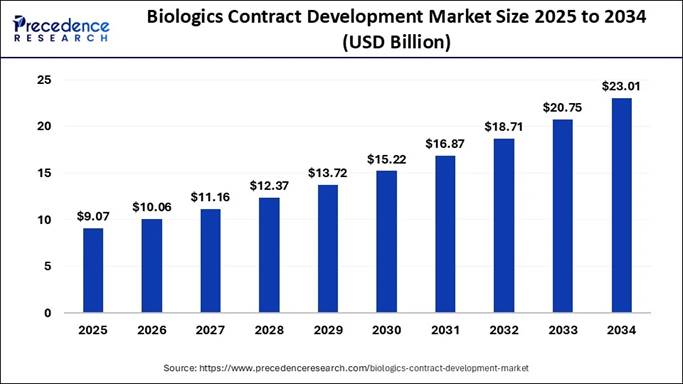
According to Precedence Research, the global biologics contract development market size is expected to surpass approximately USD 23.01 billion by 2034, fueled by outsourcing demand, oncology therapeutics, and personalized medicine.
The global biologics contract development market size accounted for USD 9.07 billion in 2025 and is expected to grow from USD 10.06 billion in 2025 to approximately USD 23.01 billion by 2034. The market is representing a double-digit compounding annual growth rate (CAGR) of 10.90% from 2025 to 2034.
The biologics contract development market is an important part of the development of complicated procedures of drug development and production methods. Genetically engineered proteins sourced from human gene technologies, referred to as biologics, are designed to target immune cascades and vary extensively to cover several products, including gene therapy, vaccines, blood components, and recombinant proteins. The growing demand for personalized or individualized medicine, soaring cases of chronic and genetic ailments, and the fast-tracking of cutting-edge biologic treatments.
Note: This
report is readily available for immediate delivery. We can review it with you
in a meeting to ensure data reliability and quality for decision-making.
📥 Download Sample Pages
for Informed Decision-Making 👉 https://www.precedenceresearch.com/sample/3056
Biologics Contract
Development Market Key Highlights: 🔹In terms of revenue, the
biologics contract development market was calculated at USD 8.18 billion in
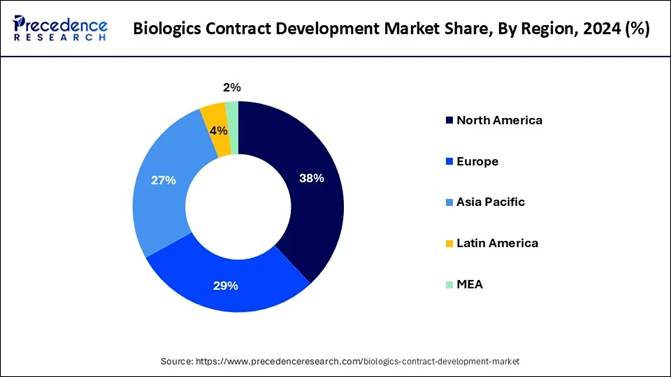
2024. 🔹It is anticipated to reach USD 23.01 billion by 2034. 🔹The North
America accounted for the largest market share of 38% in 2024. 🔹Asia Pacific is
expected to grow at a notable CAGR during the projected period. 🔹By Indication,
the oncology segment
captured the biggest market share in 2024. 🔹By Indication,
the immunological disorders segment is expected to expand at the fastest CAGR
in the coming years 🔹By product service, the
process development segment contributed the highest market share in 2024. 🔹By product service, the
cell line development segment is expected to expand at a rapid pace in the
coming years. 🔹By source, the mammalian segment generated the major
market share in 2024. Biologics Contract
Development Market Overview and Industry Potential Outsourcing and Innovation
Boost Biologics Development Market The increasing tendency of pharmaceutical and biopharmaceutical companies to outsource
their production can be considered one of the leading factors in the biologics
contract development market. This strategy also provides an opportunity to
access high-tech platforms and products, unique facilities, and regulatory
expertise that are essential to the complicated development of biologics,
including gene therapies, monoclonal antibodies, and vaccines. Outsourcing to biologics CDMOs, as
pharmaceutical companies focus on efficiency, cost-effectiveness, and
innovation, is projected to remain a robust growth driver of the industry. Major Trends in Biologics
Contract Development Market: ➡️ Rising Demand for Biologics:
Increasing
prevalence of chronic diseases and the shift toward biologic therapies is
driving the need for specialized development services. ➡️ Expansion of Outsourcing: Biopharmaceutical companies
are increasingly outsourcing development stages to reduce cost, time, and
complexity. ➡️ Technological Advancements:
Adoption of artificial intelligence (AI), automation, and
high-throughput screening in biologics development is accelerating timelines
and enhancing accuracy. ➡️ Growth in Monoclonal
Antibody Development: High
demand for monoclonal antibodies is fueling
contract development projects, especially in oncology and
autoimmune diseases. ➡️ Focus on Biosimilars: As patents expire, the demand
for biosimilar development services is
increasing,
creating opportunities for CDMOs. ➡️ Emergence of Small and
Virtual Biotech Firms: Smaller
biotech firms, often lacking in-house capabilities, are increasingly relying on
contract development partners. Mergers and Collaborations
Strengthen Biologics Contract Development Capabilities The mergers and alliances are significantly
helping to fortify the potential of the biologics contract development market.
Mergers and acquisitions allow CDMOs to increase their service offerings,
incorporate advanced technologies, and offer end-to-end capabilities, including
drug development to large-scale commercial production. Through these
partnerships, companies are better positioned to speed up time-to-market, to
mitigate risks, as well as address the increasing global demand for innovative
biologics. High Production Costs
Challenge the Biologics Contract Development Market Biologics are complex and complicated, and
all these factors make it even more expensive; they need advanced manufacturing
facilities, have intricate bio-processing equipment, and regulatory compliance.
The small and mid-sized companies, especially, encounter financial limitations
when intensifying outsourcing with CDMOs since the price of expertise and
quality control may prove to be too high. This has resulted in high capital
demand for biologics development and little adaptability in production, as a major
inhibiting factor in the market. Scope of Biologics
Contract Development Market
Report Attributes Key Statistics Market Size in 2024 USD 8.18 Billion Market Size in 2025 USD 9.07 Billion Market Size in 2031 USD 16.87 Billion Market Size by 2034 USD 23.01 Billion Growth Rate 2025 to 2034 CAGR of 10.89% Leading Region in 2024 North America Base Year 2024 Forecast Period 2025 to 2034 Segments Covered Source, Product Service, Indication, and Region Regions Covered North America, Europe, Asia-Pacific, Latin America, and Middle
East & Africa
➡️ Become a valued research
partner with us ☎ https://www.precedenceresearch.com/schedule-meeting Biologics Contract
Development Market Key Regional Analysis: How Big is the U.S.
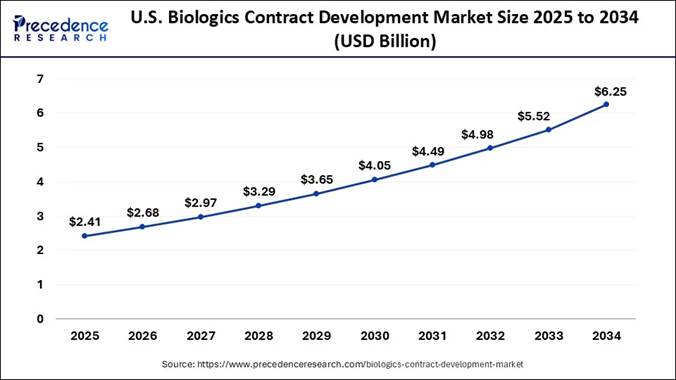
Biologics Contract Development Market? According to Precedence Research, the U.S.
biologics contract development market size has been evaluated at USD 2.41
billion in 2025 and is expected to exceed USD 6.25 billion by 2034. The market
is projected to grow at a notable CAGR of 11.11% from 2025 to 2034. North America held the
dominant share of the biologics contract development market in 2024, due to the
high industry and the increasing healthcare needs. This region is taking
advantage of the increasing incidence of chronic conditions that include
cancer, diabetes, and autoimmune diseases, contributing to the increased demand
for advanced biologics. Also, the major presence of key CDMO industry players,
well-developed infrastructure, and investments in research and development
facilitate the region's dominance. The region is also the leader in new drug
approvals, providing an opportunity to produce and commercialize biologics. What’s Driving Asia
Pacific’s Surge in Biologics Contract Development Market? Asia Pacific is expected
to expand notably during the forecast period, supported by an increasing
number of medical investments, affordable clinical trials, and affordable
medical expertise. India, China, and South Korea have become one of the most
preferred countries in clinical research with the availability of good
infrastructure, growing pharmaceutical industries, and supportive government
policy towards encouraging outsourcing and foreign investment. Biologics Contract
Development Market Segmentation Analysis: By Source Analysis: Why Did the Mammalian
Segment Dominate the Biologics Contract Development Market in 2024? The mammalian segment
dominated the market with the largest share in 2024, attributed to its high
application level in the production of complex biologics like synthetic
hormones, monoclonal antibodies, and therapeutic enzymes. The mammalian
cell cultures are important
in the production of viral vaccines, which are critical in fighting infectious
diseases in the world. As new forms of chronic illnesses and the need to access
new forms of advanced biologics are on the rise, the use of mammalian systems
results in the gold standard of biopharmaceutical manufacturing. The microbial segment is
the second-largest segment in 2023 and is projected to grow steadily in the
coming years, driven by its high productivity, affordability, and the growing
list of applications. In the production of recombinant proteins, including
insulin, antibody fragments, enzymes, and vaccines, microbial systems –
especially bacteria and yeast are commonly used. Pharmaceutical and
biotechnology companies are interested in them due to their affordability in
the provision of high-volume production. The growing use of biologics in all
therapeutic areas has also enhanced the demand for microbial-based contract development.
By Product Service
Analysis: How Does the Process
Development Segment Maintain Its Dominance in the Current Industry? The process development segment
held the largest share of the market in 2024, driven by the critical
potential in assuring the efficiency, scalable, and high-quality production of
biologic products. There are crucial techniques used in the biologics process
development, and they include upstream and downstream processing. Upstream
process engineering focuses on cell line expression and bioreactor hardware
variable operating conditions to obtain the desired optimal yield and
efficiency. Conversely, downstream processing is a technique that deals with
the purification and isolation of the biologics from host cell proteins to render the
product safe and efficacious. The cell line development
segment is expected to expand at a rapid pace in the coming years because of the
rising demand for recombinant proteins, monoclonal antibodies, and complex
biologics to treat chronic and genetic diseases. High-expression
biopharmaceutical proteins are important using recombinant cell lines that
allow the scalability of drug development and cost-effective drug development.
The increasing incidence of cancer, autoimmune diseases, and other chronic
disorders has led to an augmented demand for innovative protein-based therapies,
creating the necessity to have quality cell line development services. By Indication Analysis: Why Did the Oncology
Segment Dominate the Biologics Contract Development Market in 2024? The immunological
disorders segment is expected to expand at the fastest CAGR in the coming years, due to the
increasing incidence of autoimmune disorders like arthritis, lupus, and
rheumatologic disorders. The rising awareness among patients regarding the
disorders has enhanced the need to know more about early diagnosis, prevention,
and effective treatment of these disorders. Biologics are highly useful in the treatment
of autoimmune conditions that control abnormal immune responses and exert a
specific therapeutic effect. Key Companies and Market
Share Insights The top players in the biologics contract
development market are the Lonza Group, WuXi AppTec, and Samsung Biologics.
Lonza has the benefit of worldwide expertise with biomanufacturing in both the
clinical and commercial space, and WuXi AppTec can integrate all services and
provide flexible outsourcing. Prominent competing companies such as
Fujifilm Diosynth Biotechnologies, Thermo Fisher Scientific, and AGC Biologics
are expanding their potential through internationalization, innovativeness, and
strategic alliances, which are chief factors in establishing growth and
competitiveness within the fields of biologics CDMOs. Top Companies in Biologics
Contract Development Market & Their Contribution ➢ Abzena Ltd – Provides fully
integrated solutions for biologic drug discovery, development, and
manufacturing with strong capabilities in antibody engineering. ➢ AGC Biologics – Offers comprehensive
biologics CDMO services including cell line development, process development,
and clinical to commercial manufacturing. ➢ Bionova Scientific, Inc. – Specializes in cell line
and process development for complex biologics, particularly monoclonal
antibodies. ➢ BioXcellence – A division of Merck KGaA
delivering high-quality biologics development and GMP manufacturing services. ➢ Curia Global, Inc. – Delivers biologics
development and manufacturing solutions leveraging advanced technologies and
global infrastructure. ➢ Fujifilm Diosynth
Biotechnologies
– Recognized for its expertise in microbial and mammalian biologics development
and large-scale manufacturing. ➢ Genscript – Offers biologics
discovery and development services with a focus on gene synthesis, protein
engineering, and cell therapy support. ➢ KBI Biopharma – Provides end-to-end
biologics development and manufacturing, including analytical services and
formulation development. ➢ STC Biologics – Specializes in
biosimilar and biologic formulation development, with strong regulatory and
analytical support. ➢ Thermo Fisher Scientific
Inc.
– Offers comprehensive CDMO services for biologics through its Patheon
division, with global manufacturing facilities. ➢ WuXi Biologics – One of the largest
biologics CDMOs globally, providing integrated development and manufacturing
platforms from concept to commercial. What is Going Around the
Globe? 🔹In October
2024, Samsung Biologics launched S-HiCon, a high-concentration formulation
platform to help develop and manufacture higher-dose biopharmaceuticals. This
breakthrough is able to improve delivery efficiency with an improved solution
to complex biologics.
🔹In May 2024,
AGC Biologics, in partnership with BioConnection, established end-to-end
biopharmaceutical development and manufacturing capabilities. The partnership
will aim at combining the drug substance manufacturing with aseptic filling
into vials and syringes as clinical and commercial products.
🔹In June 2024,
Abzena partnered with Argonaut Manufacturing Services to deliver a complete
drug substance and drug product manufacturing solution. The alliance boosts the
availability of biopharmaceutical organizations to efficient development,
manufacture, and bioconjugate production. Biologics Contract
Development Market Segmentation: By Source 🔹 Microbial 🔹 Mammalian 🔹 Others By Product
Service 🔹 Cell Line
Development • Microbial • Mammalian • Others 🔹 Process Development • Upstream → Microbial → Mammalian → Others • Downstream → Impurity,
isolation, & identification → Physicochemical
characterization → Pharmaceutical
analysis → Others • By Product → MABs → Recombinant
proteins → Others 🔹Others By Indication 🔹 Oncology 🔹 Immunological
disorders 🔹 Cardiovascular
disorders 🔹 Hematological
disorders 🔹 Others By Geography 🔹 North America 🔹 Europe 🔹 Asia-Pacific 🔹 Latin America 🔹 Middle East and
Africa Thanks for reading you can also get individual
chapter-wise sections or region-wise report versions such as North America,
Europe, or Asia Pacific. ⏳ Don’t Miss Out!
| ⚡ Instant Access to
This Exclusive Report 👉 https://www.precedenceresearch.com/checkout/3056 You can place an order or
ask any questions, please feel free to contact at sales@precedenceresearch.com | +1
804 441 9344 Stay
Ahead with Precedence Research Subscriptions Unlock exclusive access to
powerful market intelligence, real-time data, and forward-looking insights,
tailored to your business. From trend tracking to competitive analysis, our
subscription plans keep you informed, agile, and ahead of the curve. Browse Our Subscription
Plans@ https://www.precedenceresearch.com/get-a-subscription About Us Precedence Research is a
global market intelligence and consulting powerhouse, dedicated to unlocking
deep strategic insights that drive innovation and transformation. With a laser focus
on the dynamic world of life sciences, we specialize in decoding the
complexities of cell and gene therapy, drug development, and oncology markets, helping our clients stay
ahead in some of the most cutting-edge and high-stakes domains in healthcare.
Our expertise spans across the biotech and pharmaceutical ecosystem, serving
innovators, investors, and institutions that are redefining what’s possible in regenerative
medicine,
cancer care, precision therapeutics, and beyond. Web: https://www.precedenceresearch.com Our Trusted Data Partners: Towards Healthcare | Nova One Advisor Get Recent News 👉 https://www.precedenceresearch.com/news For Latest Update Follow
Us: LinkedIn | Medium | Facebook | Twitter ___________________________________________________________________________ Frequently Asked Questions: ✚ What is the
biologics contract development market size? ➢ The global
biologics contract development market size is expected to increase USD 23.01
billion by 2034 from USD 8.18 billion in 2024. ✚ What will be
the CAGR of global biologics contract development market? ➢ The global
biologics contract development market will register growth rate of 10.90%
between 2025 and 2034. ✚ Who are the
prominent players operating in the biologics contract development market? ➢ The major
players operating in the biologics contract development market are Abzena Ltd,
AGC Biologics, Bionova Scientific, Inc., BioXcellence, Curia Global, Inc., Fujifilm
Diosynth Biotechnologies, Genscript, KBI Biopharma, STC Biologics, Thermo
Fisher Scientific Inc., WuXi Biologics, Boehringer Ingelheim Group, Samsung
Biologics, Lonza Group, and Others. ✚ Which are the
driving factors of the biologics contract development market? ➢ The driving
factors of the biologics contract development market are the rising adoption of
advanced technologies for biological production, increasing R&D activities
by biopharma and pharma firms, rising the number of small and medium pharmaceutical
manufacturing companies, increasing demand for pharmaceutical drugs, and
increasing merger and acquisition activities. ✚ Which region
will lead the global biologics contract development market? ➢ North America
region will lead the global biologics contract development market during the
forecast period 2025 to 2034. ➡️ Explore More Market
Intelligence from Precedence Research: 💡 Next
Generation Biologics Market: Explore how advanced biologics are
reshaping therapies and patient outcomes 💡 Biotechnology
Contract Manufacturing Market: Understand outsourcing trends
driving innovation and large-scale biologics production 💡 Fibroblast
Growth Factors Market: See how regenerative medicine and wound healing
therapies are boosting demand 💡 Contract
Manufacturing Partnerships Market: Analyze collaborations fueling
biopharma efficiency and global scalability 💡 CDMO Market Insights: Gain perspective on how
CDMOs are transforming the drug development landscape 💡 Cell
and Gene Therapy CDMO Market: Discover why cell and gene therapy
outsourcing is accelerating clinical pipelines 💡 Pharmaceutical
CDMO Market:
Track how pharma companies rely on CDMOs for speed, quality, and compliance 💡 U.S.
Pharmaceutical CDMO Market: Explore why the U.S. leads in
advanced outsourcing and biologics partnerships 💡 Topical
Drugs CDMO Market: Understand rising demand for CDMOs in dermatology and
transdermal drug delivery 💡 Europe
Pharmaceutical CDMO Market: See how European CDMOs are scaling
biologics and complex formulations 💡 mRNA
Therapeutics CDMO Market: Discover outsourcing opportunities
fueled by rapid mRNA-based therapy adoption 💡 Pharmaceutical
CDMO for Formulations Market: Explore CDMO capabilities in drug
formulation, stability, and delivery solutions 💡 Investigational
New Drug CDMO Market: Learn how CDMOs accelerate IND applications and
clinical trial readiness 💡 Contract
Development and Manufacturing Organization Outsourcing Market: See how outsourcing is
shaping global drug development strategies 💡 Anti-Inflammatory
Biologics Market:
Analyze how biologics are redefining treatment for autoimmune and chronic
diseases 💡 Vaccine
Contract Manufacturing Market: Track how outsourcing supports
vaccine scale-up and global immunization efforts 💡 Drug
Discovery Services Market: Understand how AI and outsourcing
accelerate early-stage drug innovation 💡 Biopharmaceutical
CMO and CRO Market: Explore synergies between CMOs and CROs driving
efficiency in biopharma 💡 Small
Molecule Innovator CDMO Market: Learn how CDMOs support novel small
molecule development and commercialization 💡 Healthcare
Contract Manufacturing Market: See how outsourcing strengthens
medical devices, diagnostics, and pharma supply chains 💡 Drug
Discovery Outsourcing Market: Discover how outsourcing reduces R&D
costs and accelerates therapeutic pipelines