Neurocrine Biosciences, Inc. announced that it will present new Phase 2 data on the use of crinecerfont in adolescent patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency, which demonstrated substantial reductions in adrenal androgens and androgen precursors.
- Substantial Reductions in Key Hormones and Hormone Precursors Observed After 14 Days of Crinecerfont Treatment in Adolescents with Classic CAH
- Two Phase 3 Global Registrational Studies Currently Underway in Pediatric (2–17 years of age) and Adult (18 years of age and older) Patients with Classic CAH
SAN DIEGO, June 13, 2022 /PRNewswire/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced that it will present new Phase 2 data on the use of crinecerfont in adolescent patients with classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21-OHD), which demonstrated substantial reductions in adrenal androgens and androgen precursors. These data will be shared as an oral presentation by Ron S. Newfield, M.D., University of California, San Diego and Rady Children's Hospital, San Diego on June 13 from 11:00 a.m.–12:30 p.m. ET (Presentation #OR18) at ENDO 2022, the Endocrine Society's 104th annual meeting in Atlanta from June 11–14. The company also presented data highlighting patient preferences and treatment patterns in classic CAH in Hall A1 on Saturday, June 11.
"Management of classic CAH can be particularly challenging in adolescents due to the hormonal changes that take place during puberty," said Eiry W. Roberts, M.D., Chief Medical Officer at Neurocrine Biosciences. "There are no non-steroidal treatment options for CAH patients. The Phase 2 data for crinecerfont that we are presenting at ENDO 2022 demonstrate a reduction in levels of key hormones and hormone precursors at a critical transition stage in a young person's life. High levels of androgen and adrenocorticotropic hormone, or ACTH, in adolescents can lead to virilization and menstrual irregularities in females and testicular adrenal rest tumors in males, which can result in fertility problems for both sexes in adulthood. We are committed to advancing this potential new treatment option for people living with this condition and are currently enrolling children, adolescents, and adults in two Phase 3 clinical studies of crinecerfont in classic CAH at sites in the U.S., Canada, and Europe."
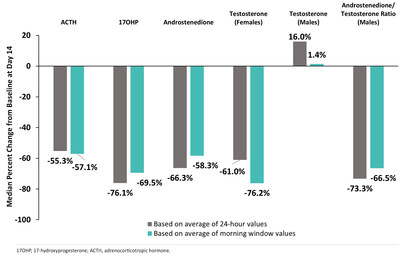
The 14-day, open-label Phase 2 study evaluated the effect of crinecerfont in eight adolescents 14–17 years of age (three males, five females) with classic CAH due to 21-OHD (Figure 1). Median percent reductions from baseline to Day 14 for 24-hour measurements of ACTH, 17-hydroxyprogesterone (17-OHP), and androstenedione (males and females), and androstenedione/testosterone ratio (males) ranged from -55.3% to -76.1%, while morning window measurements (i.e., the average of the two samples collected at 7:00 a.m. and 10:00 a.m.) ranged from -57.1% to -76.2%. Levels of these hormones and hormone precursors are often elevated in patients with classic CAH. Additionally, a greater than 50% reduction from baseline for ACTH, 17-OHP, and androstenedione (males and females), testosterone (females), and androstenedione/testosterone ratio (males) was achieved by 63%, 75%, 50%, 60%, and 67% of participants, respectively.
Crinecerfont was generally well tolerated, with no serious adverse events or discontinuations due to adverse events. All treatment-emergent adverse events were assessed as mild, with two adverse events (headache and dizziness) assessed as possibly related by the study investigator.
"Treatment of children and adolescents with classic CAH is challenging. We have relied on steroids, specifically glucocorticoids, for over 50 years, often resulting in under- or over-treatment, which can result in lower adult height and other health problems," said Ron S. Newfield, M.D., University of California, San Diego and Rady Children's Hospital. "I am honored to be a part of a dedicated team of investigators, looking at a new potential paradigm in treating classic CAH. We are eagerly awaiting the results of the larger and longer-term study that is under way. I am grateful to all the adolescents that participated in this intensive and important study."
Neurocrine Biosciences is developing crinecerfont, an investigational, oral, non-steroidal, selective corticotropin-releasing factor type 1 receptor (CRF1) antagonist for the treatment of classic CAH due to 21-OHD. Neurocrine Biosciences is currently conducting two Phase 3 global registrational studies of crinecerfont in adults (18 years of age and older) and children and adolescents (2 to17 years of age) with classic CAH, called the CAHtalyst™ and CAHtalyst™ Pediatric studies, respectively. As part of the CAHtalyst clinical trial program, participants who complete these trials will be able to continue to receive crinecerfont as part of an open-label extension.
Additional Presentations:
Saturday, June 11 (1:00 p.m.– 3:00 p.m.) in Hall A1
- Patient Preference Research: Preferred Adjunctive Medication Attributes of Adult Patients with Classic Congenital Adrenal Hyperplasia (Poster #PSAT097)
- Examination of Treatment Patterns in Patients with Classic Congenital Adrenal Hyperplasia (CAH) Compared to Treatment Guidelines (Poster #PSAT095)
A full list of all abstracts being presented by Neurocrine Biosciences at ENDO 2022 is available here.
About Classic Congenital Adrenal Hyperplasia (CAH)
Congenital adrenal hyperplasia (CAH) refers to a group of genetic conditions that result in an enzyme deficiency that alters the production of adrenal hormones. Approximately 95% of CAH cases are caused by a mutation that leads to deficiency of the enzyme 21-hydroxylase. In classic CAH, severe deficiency of this enzyme leads to an inability of the adrenal glands to produce cortisol and, in approximately 75% of cases, aldosterone. If left untreated, classic CAH can result in salt wasting, dehydration, and even death. Even with glucocorticoid treatment, high levels of adrenocorticotropic hormone (ACTH) from the pituitary gland result in excess androgen production leading to virilization and menstrual irregularities in females. Both males and females with classic CAH can experience problems with growth and development in childhood including early puberty, short stature or height below genetic potential, and fertility problems in adulthood.
There are currently no non-steroidal treatments approved by the U.S. Food and Drug Administration (FDA) for classic CAH. Glucocorticoids, the current standard of care, are used to correct the endogenous cortisol deficiency and to try to reduce the high ACTH levels and androgen excess. However, the dose of steroid use required to try to control androgen excess is generally well above the normal physiological level of cortisol, and the chronic duration of high steroid dose administration can result in serious and common complications of steroid excess, including metabolic abnormalities, increased cardiovascular risk, bone loss, fractures, growth impairment, and increased infection risk.
To learn more about CAH, click here.
About Crinecerfont
Crinecerfont is an investigational, oral, nonsteroidal, selective corticotropin-releasing factor type 1 receptor (CRF1) antagonist under evaluation for the treatment of classic CAH due to 21-hydroxylase deficiency (21-OHD). Antagonism of CRF1 receptors in the pituitary has been shown to decrease ACTH levels, which in turn could decrease the production of adrenal androgens and potentially the symptoms associated with classic CAH. Research also suggests that lowering androgen levels may enable lower, more physiologic dosing of glucocorticoids and thus potentially reduce the complications associated with long-term exposure to greater than normal glucocorticoid doses in patients with classic CAH.
To learn more about crinecerfont, click here.
About CAHtalyst™ Studies
Neurocrine Biosciences is currently conducting two Phase 3 global registrational studies of crinecerfont in adults (18 years of age and older) and children and adolescents (ages 2 to 17 years of age) with classic CAH.
For more information about the adult CAHtalyst™ Phase 3 study, please visit cahtalyst.cahstudies.com and ClinicalTrials.gov.
For more information about the pediatric CAHtalyst™ Phase 3 study, please visit cahtalystpeds.cahstudies.com and ClinicalTrials.gov.
As part of the CAHtalyst™ clinical trial program, participants who complete these trials will be able to continue to receive crinecerfont as part of an open-label extension.
About Neurocrine Biosciences
Neurocrine Biosciences is a neuroscience-focused, biopharmaceutical company with a simple purpose: to relieve suffering for people with great needs, but few options. We are dedicated to discovering and developing life-changing treatments for patients with under-addressed neurological, neuroendocrine, and neuropsychiatric disorders. The company's diverse portfolio includes FDA-approved treatments for tardive dyskinesia, Parkinson's disease, endometriosis* and uterine fibroids*, as well as over a dozen mid- to late-stage clinical programs in multiple therapeutic areas. For three decades, we have applied our unique insight into neuroscience and the interconnections between brain and body systems to treat complex conditions. We relentlessly pursue medicines to ease the burden of debilitating diseases and disorders, because you deserve brave science. For more information, visit neurocrine.com, and follow the company on LinkedIn, Twitter, and Facebook. (*in collaboration with AbbVie).
Neurocrine, the Neurocrine logo, and CAHtalyst are registered trademarks of Neurocrine Biosciences, Inc.
Neurocrine Biosciences Forward-Looking Statement
In addition to historical facts, this press release contains forward-looking statements that involve a number of risks and uncertainties. These statements include, but are not limited to, statements regarding the potential benefits of crinecerfont to patients and future clinical development plans. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements include: our future financial and operating performance; risks and uncertainties associated with the scale and duration of the COVID-19 pandemic and resulting global, national, and local disruptions, the risk that crinecerfont will not be found to be safe and/or effective or may not prove to be beneficial to patients; that development activities for crinecerfont may not be completed on time or at all; risks that clinical development activities may be delayed for regulatory or other reasons, may not be successful or replicate previous and/or interim clinical trial results, or may not be predictive of real-world results or of results in subsequent clinical trials; risks that regulatory submissions for crinecerfont may not occur or be submitted in a timely manner; risks that crinecerfont may not obtain regulatory approvals; or that the U.S. Food and Drug Administration or regulatory authorities outside the U.S. may make adverse decisions regarding crinecerfont; and other risks described in the Company's periodic reports filed with the Securities and Exchange Commission, including without limitation the Company's quarterly report on Form 10-Q for the quarter ended March 31, 2022. Neurocrine Biosciences disclaims any obligation to update the statements contained in this press release after the date hereof.
©2022 Neurocrine Biosciences, Inc. All Rights Reserved.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-presents-data-on-treatment-of-adolescent-patients-with-classic-congenital-adrenal-hyperplasia-at-endo-2022-301565971.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-presents-data-on-treatment-of-adolescent-patients-with-classic-congenital-adrenal-hyperplasia-at-endo-2022-301565971.html
SOURCE Neurocrine Biosciences, Inc.
Company Codes: NASDAQ-NMS:NBIX