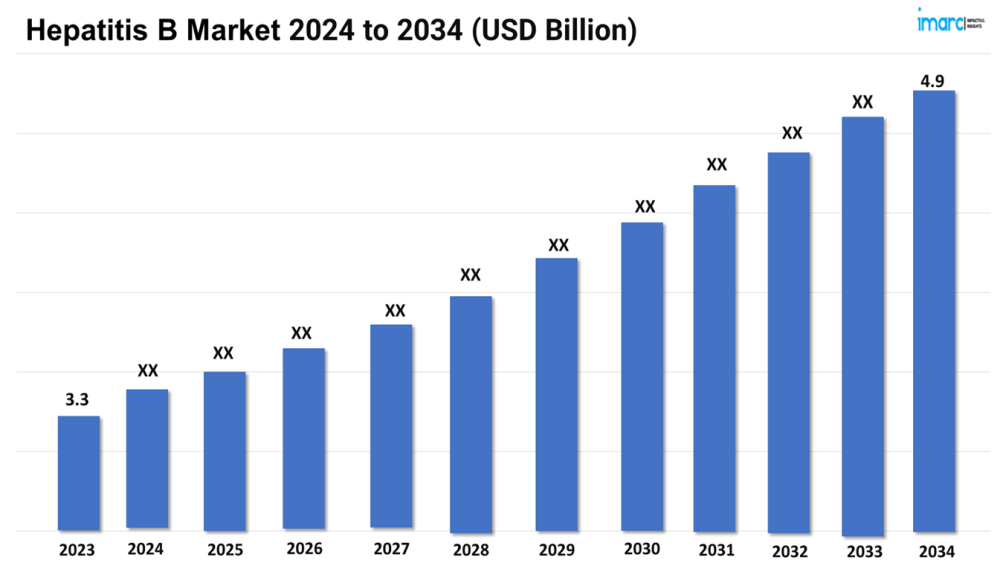
The hepatitis B market size reached a value of USD 3.3 Billion in 2023. Looking forward, the market is expected to reach USD 4.9 Billion by 2034, exhibiting a growth rate (CAGR) of 3.83% during 2024-2034.
The market is driven by the introduction of new antiviral drugs that offer higher precision as well as fewer side effects. Additionally, there is a rising emphasis on long-term management strategies and combination therapies to prevent drug resistance, which further strengthens the market growth.
Advancements in Antiviral Therapies: Driving the Hepatitis B Market
One of the major trends revolutionizing the hepatitis B market is advancements in antiviral therapies, offering more effective along with safer treatment alternatives for patients. Recent years have experienced the launch of new antiviral drugs that target the Hepatitis B virus (HBV) more efficiently. tenofovir alafenamide (TAF), a prodrug of tenofovir. TAF has shown superior efficacy and a better safety profile compared to its predecessor, tenofovir disoproxil fumarate (TDF). TAF is effective at lower doses, resulting in reduced renal and bone toxicity, which are significant concerns with long-term HBV treatment. Another major drug, entecavir, continues to be a cornerstone in HBV therapy due to its potent antiviral activity and high barrier to resistance. These drugs have significantly improved the management of chronic Hepatitis B, allowing patients to achieve better viral suppression and reduced risk of liver complications.
Request a PDF Sample Report: https://www.imarcgroup.com/hepatitis-b-market/requestsample
Moreover, combination therapies are becoming increasingly prominent in the Hepatitis B treatment paradigm. Combining antiviral agents with immune-modulating treatments aims to enhance the therapeutic effects and prevent the emergence of drug resistance. For instance, research is ongoing into combining TAF or entecavir with pegylated interferon, an immune system booster, to achieve higher rates of sustained viral response and potential functional cures. Additionally, innovative approaches like RNA interference (RNAi) are being explored to target HBV replication. The drug candidate JNJ-3989, an RNAi therapeutic, has shown promise in reducing HBV surface antigen levels significantly, indicating its potential as part of combination therapy to achieve better clinical outcomes. These advancements underscore a dynamic period in the Hepatitis B market, driven by the need for more effective and safer treatments. With ongoing research and development, the future of HBV therapy looks promising, offering hope for improved patient management and the potential for long-term control or even a cure for Hepatitis B.
Focus on Combination Therapies: Contributing to Market Expansion
The hepatitis B market is increasingly focusing on combination therapies, aiming to enhance treatment efficacy and overcome the limitations of monotherapy. Combination therapies involve the simultaneous use of multiple drugs with different mechanisms of action to achieve better viral suppression, reduce the risk of drug resistance, and improve patient outcomes. For instance, combining nucleos(t)ide analogs like tenofovir alafenamide (TAF) or entecavir with immune-modulating agents such as pegylated interferon has shown promising results. Pegylated interferon boosts the immune system's ability to fight the virus, while nucleos(t)ide analogs inhibit viral replication. This dual approach not only enhances the antiviral effect but also increases the likelihood of achieving a sustained virological response (SVR), which is a crucial indicator of long-term treatment success.
Recent clinical trials have highlighted the potential of innovative combination therapies. For example, a study involving the combination of TAF with the immune modulator pegylated interferon demonstrated higher rates of HBeAg seroconversion and HBsAg loss compared to monotherapy. This is significant because HBsAg loss is considered a functional cure for Hepatitis B, leading to a substantial reduction in the risk of liver-related complications and improving the patient's quality of life. Furthermore, the exploration of combining antiviral therapies with novel agents like RNA interference (RNAi) therapeutics is gaining momentum. The drug candidate JNJ-3989, when used in combination with nucleos(t)ide analogs, has shown a substantial reduction in HBsAg levels, indicating its potential to enhance the overall antiviral response. These developments underscore the importance of combination therapies in the future of Hepatitis B treatment. By integrating different therapeutic mechanisms, these approaches aim to achieve more robust and durable responses, ultimately improving clinical outcomes and offering new hope for patients. As research progresses, combination therapies are expected to play a pivotal role in managing chronic Hepatitis B, potentially leading to more effective and curative treatment regimens.
Innovations in Gene Editing and Vaccination:
Innovations in gene editing and vaccination are transforming the Hepatitis B market, offering promising new avenues for prevention and treatment. Gene editing technologies, particularly CRISPR-Cas9, have emerged as groundbreaking tools with the potential to cure Hepatitis B by targeting and eliminating the viral genome within infected cells. CRISPR-Cas9 works by precisely cutting the DNA at specific sites, allowing for the removal or alteration of the HBV DNA integrated into the host genome. Recent preclinical studies have demonstrated the ability of CRISPR-Cas9 to significantly reduce HBV replication and gene expression in liver cells. For instance, researchers have successfully used CRISPR-Cas9 to target and disrupt the covalently closed circular DNA (cccDNA) of HBV, which is critical for the virus's persistence in the liver. These advancements suggest that gene editing could potentially provide a functional cure for chronic Hepatitis B, offering a transformative impact on patient outcomes.
On the vaccination front, there have been significant strides in developing more effective vaccines to prevent Hepatitis B infection. Current HBV vaccines are highly effective but require a series of doses to achieve long-term immunity. Innovations in vaccine technology aim to enhance the immunogenicity and convenience of these vaccines. For example, novel adjuvants and delivery systems are being explored to boost the immune response and reduce the number of doses required. A promising development is the use of virus-like particles (VLPs) in HBV vaccines, which mimic the natural structure of the virus without being infectious, thereby eliciting a strong immune response. Additionally, efforts are underway to develop therapeutic vaccines that can help clear the virus in chronically infected individuals by stimulating a robust and targeted immune response against HBV-infected cells. These advancements in gene editing and vaccination are poised to revolutionize the Hepatitis B market. By addressing both prevention and treatment, they offer a comprehensive approach to managing and potentially eradicating the disease. The continued research and development in these areas hold great promise for achieving long-term control and even cure of Hepatitis B, significantly improving the lives of millions affected by this chronic infection.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7245&method=587
Leading Companies in the Hepatitis B Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global hepatitis B market, several leading companies in the hepatitis B market are at the forefront of developing innovative therapies and vaccines to combat this chronic infection. Some of the major players include Dynavax Technologies, VBI Vaccines, and Bristol-Myers Squibb. These companies play a pivotal role in the hepatitis B market, driving advancements in both preventive and therapeutic measures.
Dynavax Technologies' ongoing efforts to expand the use and recognition of Heplisav-B. The vaccine has been gaining traction in various healthcare settings, including hospitals, pharmacies, and occupational health programs, due to its efficacy and simplified dosing regimen. Studies have shown that Heplisav-B induces a higher seroprotection rate in adults compared to other hepatitis B vaccines, particularly in populations that are harder to immunize, such as older adults and those with diabetes.
Moreover, VBI Vaccines is an influential player in the Hepatitis B market, primarily known for its vaccine PreHevbrio (Hepatitis B vaccine recombinant). PreHevbrio is designed to offer comprehensive protection against all known subtypes of the Hepatitis B virus, addressing a critical need for effective and broad-spectrum immunization.
Apart from this, Bristol-Myers Squibb has been focusing on optimizing the use of Baraclude through various research initiatives and clinical studies. One of the significant advancements has been the investigation into combination therapies involving Baraclude. Studies are exploring the efficacy of combining Baraclude with other antiviral agents or immune modulators to enhance treatment outcomes. This approach aims to achieve higher rates of sustained virological response (SVR) and reduce the risk of drug resistance, which remains a challenge in long-term HBV treatment.
Request for customization: https://www.imarcgroup.com/request?type=report&id=7245&flag=E
Regional Analysis:
The major markets for hepatitis B include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for hepatitis B while also representing the biggest market for its treatment. This can be attributed to the increasing prevalence of hepatitis B virus (HBV) infections and the rising awareness of the disease's impact on public health.
Moreover, according to the Centers for Disease Control and Prevention (CDC), approximately 862,000 people in the United States are living with chronic HBV infection. The growing number of chronic HBV cases necessitates robust diagnosis, management, and treatment strategies, thereby driving the market.
Besides this, the heightened awareness has led to increased efforts in early diagnosis and vaccination programs across the country. Government initiatives and healthcare policies promoting routine HBV screening and immunization, especially among high-risk populations, are further propelling the market.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
This report offers a comprehensive analysis of current hepatitis B marketed drugs and late-stage pipeline drugs.
In-Market Drugs
IMARC Group Offer Other Reports:
Cannabis Infused Edible Products Market: The global cannabis infused edible products market size reached US$ 19.5 Billion in 2023, and projected to reach US$ 63.2 Billion by 2032, exhibiting a growth rate (CAGR) of 13.97% during the forecast period from 2024 to 2032.
Cardiac Prosthetic Devices Market: The global cardiac prosthetic devices market size reached US$ 6.7 Billion in 2023, and projected to reach US$ 12.3 Billion by 2032, exhibiting a growth rate (CAGR) of 6.8% during the forecast period from 2024 to 2032.
Foot and Mouth Disease (FMD) Vaccine Market: The global foot and mouth disease vaccine market size reached US$ 2,129.0 Million in 2023, and projected to reach US$ 3,861.8 Million by 2032, exhibiting a growth rate (CAGR) of 6.6% during the forecast period from 2024 to 2032.
Asthma Therapeutics Market: The global asthma therapeutics market size reached US$ 18 Billion in 2023, and projected to reach US$ 22.0 Billion by 2032, exhibiting a growth rate (CAGR) of 1.9% during the forecast period from 2024 to 2032.
Breath Analyzer Market; The global breath analyzer market size reached US$ 4.0 Billion in 2023, and projected to reach US$ 23.5 Billion by 2032, exhibiting a growth rate (CAGR) of 20.8% during the forecast period from 2024 to 2032.
Laser Hair Removal Market: The global laser hair removal market size is projected to exhibit a growth rate (CAGR) of 14.67% during the forecast period from 2024 to 2032.
Glioblastoma Multiforme Treatment Market: The global glioblastoma multiforme treatment market size reached US$ 2.1 Billion in 2023, and projected to reach US$ 3.9 Billion by 2032, exhibiting a growth rate (CAGR) of 6.7% during the forecast period from 2024 to 2032.
Drug Discovery Informatics Market: The global drug discovery informatics market size reached US$ 3.3 Billion in 2023, and projected to reach US$ 7.8 Billion by 2032, exhibiting a growth rate (CAGR) of 9.7% during the forecast period from 2024 to 2032.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The market is driven by the introduction of new antiviral drugs that offer higher precision as well as fewer side effects. Additionally, there is a rising emphasis on long-term management strategies and combination therapies to prevent drug resistance, which further strengthens the market growth.
Advancements in Antiviral Therapies: Driving the Hepatitis B Market
One of the major trends revolutionizing the hepatitis B market is advancements in antiviral therapies, offering more effective along with safer treatment alternatives for patients. Recent years have experienced the launch of new antiviral drugs that target the Hepatitis B virus (HBV) more efficiently. tenofovir alafenamide (TAF), a prodrug of tenofovir. TAF has shown superior efficacy and a better safety profile compared to its predecessor, tenofovir disoproxil fumarate (TDF). TAF is effective at lower doses, resulting in reduced renal and bone toxicity, which are significant concerns with long-term HBV treatment. Another major drug, entecavir, continues to be a cornerstone in HBV therapy due to its potent antiviral activity and high barrier to resistance. These drugs have significantly improved the management of chronic Hepatitis B, allowing patients to achieve better viral suppression and reduced risk of liver complications.
Request a PDF Sample Report: https://www.imarcgroup.com/hepatitis-b-market/requestsample
Moreover, combination therapies are becoming increasingly prominent in the Hepatitis B treatment paradigm. Combining antiviral agents with immune-modulating treatments aims to enhance the therapeutic effects and prevent the emergence of drug resistance. For instance, research is ongoing into combining TAF or entecavir with pegylated interferon, an immune system booster, to achieve higher rates of sustained viral response and potential functional cures. Additionally, innovative approaches like RNA interference (RNAi) are being explored to target HBV replication. The drug candidate JNJ-3989, an RNAi therapeutic, has shown promise in reducing HBV surface antigen levels significantly, indicating its potential as part of combination therapy to achieve better clinical outcomes. These advancements underscore a dynamic period in the Hepatitis B market, driven by the need for more effective and safer treatments. With ongoing research and development, the future of HBV therapy looks promising, offering hope for improved patient management and the potential for long-term control or even a cure for Hepatitis B.
Focus on Combination Therapies: Contributing to Market Expansion
The hepatitis B market is increasingly focusing on combination therapies, aiming to enhance treatment efficacy and overcome the limitations of monotherapy. Combination therapies involve the simultaneous use of multiple drugs with different mechanisms of action to achieve better viral suppression, reduce the risk of drug resistance, and improve patient outcomes. For instance, combining nucleos(t)ide analogs like tenofovir alafenamide (TAF) or entecavir with immune-modulating agents such as pegylated interferon has shown promising results. Pegylated interferon boosts the immune system's ability to fight the virus, while nucleos(t)ide analogs inhibit viral replication. This dual approach not only enhances the antiviral effect but also increases the likelihood of achieving a sustained virological response (SVR), which is a crucial indicator of long-term treatment success.
Recent clinical trials have highlighted the potential of innovative combination therapies. For example, a study involving the combination of TAF with the immune modulator pegylated interferon demonstrated higher rates of HBeAg seroconversion and HBsAg loss compared to monotherapy. This is significant because HBsAg loss is considered a functional cure for Hepatitis B, leading to a substantial reduction in the risk of liver-related complications and improving the patient's quality of life. Furthermore, the exploration of combining antiviral therapies with novel agents like RNA interference (RNAi) therapeutics is gaining momentum. The drug candidate JNJ-3989, when used in combination with nucleos(t)ide analogs, has shown a substantial reduction in HBsAg levels, indicating its potential to enhance the overall antiviral response. These developments underscore the importance of combination therapies in the future of Hepatitis B treatment. By integrating different therapeutic mechanisms, these approaches aim to achieve more robust and durable responses, ultimately improving clinical outcomes and offering new hope for patients. As research progresses, combination therapies are expected to play a pivotal role in managing chronic Hepatitis B, potentially leading to more effective and curative treatment regimens.
Innovations in Gene Editing and Vaccination:
Innovations in gene editing and vaccination are transforming the Hepatitis B market, offering promising new avenues for prevention and treatment. Gene editing technologies, particularly CRISPR-Cas9, have emerged as groundbreaking tools with the potential to cure Hepatitis B by targeting and eliminating the viral genome within infected cells. CRISPR-Cas9 works by precisely cutting the DNA at specific sites, allowing for the removal or alteration of the HBV DNA integrated into the host genome. Recent preclinical studies have demonstrated the ability of CRISPR-Cas9 to significantly reduce HBV replication and gene expression in liver cells. For instance, researchers have successfully used CRISPR-Cas9 to target and disrupt the covalently closed circular DNA (cccDNA) of HBV, which is critical for the virus's persistence in the liver. These advancements suggest that gene editing could potentially provide a functional cure for chronic Hepatitis B, offering a transformative impact on patient outcomes.
On the vaccination front, there have been significant strides in developing more effective vaccines to prevent Hepatitis B infection. Current HBV vaccines are highly effective but require a series of doses to achieve long-term immunity. Innovations in vaccine technology aim to enhance the immunogenicity and convenience of these vaccines. For example, novel adjuvants and delivery systems are being explored to boost the immune response and reduce the number of doses required. A promising development is the use of virus-like particles (VLPs) in HBV vaccines, which mimic the natural structure of the virus without being infectious, thereby eliciting a strong immune response. Additionally, efforts are underway to develop therapeutic vaccines that can help clear the virus in chronically infected individuals by stimulating a robust and targeted immune response against HBV-infected cells. These advancements in gene editing and vaccination are poised to revolutionize the Hepatitis B market. By addressing both prevention and treatment, they offer a comprehensive approach to managing and potentially eradicating the disease. The continued research and development in these areas hold great promise for achieving long-term control and even cure of Hepatitis B, significantly improving the lives of millions affected by this chronic infection.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7245&method=587
Leading Companies in the Hepatitis B Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global hepatitis B market, several leading companies in the hepatitis B market are at the forefront of developing innovative therapies and vaccines to combat this chronic infection. Some of the major players include Dynavax Technologies, VBI Vaccines, and Bristol-Myers Squibb. These companies play a pivotal role in the hepatitis B market, driving advancements in both preventive and therapeutic measures.
Dynavax Technologies' ongoing efforts to expand the use and recognition of Heplisav-B. The vaccine has been gaining traction in various healthcare settings, including hospitals, pharmacies, and occupational health programs, due to its efficacy and simplified dosing regimen. Studies have shown that Heplisav-B induces a higher seroprotection rate in adults compared to other hepatitis B vaccines, particularly in populations that are harder to immunize, such as older adults and those with diabetes.
Moreover, VBI Vaccines is an influential player in the Hepatitis B market, primarily known for its vaccine PreHevbrio (Hepatitis B vaccine recombinant). PreHevbrio is designed to offer comprehensive protection against all known subtypes of the Hepatitis B virus, addressing a critical need for effective and broad-spectrum immunization.
Apart from this, Bristol-Myers Squibb has been focusing on optimizing the use of Baraclude through various research initiatives and clinical studies. One of the significant advancements has been the investigation into combination therapies involving Baraclude. Studies are exploring the efficacy of combining Baraclude with other antiviral agents or immune modulators to enhance treatment outcomes. This approach aims to achieve higher rates of sustained virological response (SVR) and reduce the risk of drug resistance, which remains a challenge in long-term HBV treatment.
Request for customization: https://www.imarcgroup.com/request?type=report&id=7245&flag=E
Regional Analysis:
The major markets for hepatitis B include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for hepatitis B while also representing the biggest market for its treatment. This can be attributed to the increasing prevalence of hepatitis B virus (HBV) infections and the rising awareness of the disease's impact on public health.
Moreover, according to the Centers for Disease Control and Prevention (CDC), approximately 862,000 people in the United States are living with chronic HBV infection. The growing number of chronic HBV cases necessitates robust diagnosis, management, and treatment strategies, thereby driving the market.
Besides this, the heightened awareness has led to increased efforts in early diagnosis and vaccination programs across the country. Government initiatives and healthcare policies promoting routine HBV screening and immunization, especially among high-risk populations, are further propelling the market.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the hepatitis B market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the hepatitis B market
- Reimbursement scenario in the market
- In-market and pipeline drugs
This report offers a comprehensive analysis of current hepatitis B marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
IMARC Group Offer Other Reports:
Cannabis Infused Edible Products Market: The global cannabis infused edible products market size reached US$ 19.5 Billion in 2023, and projected to reach US$ 63.2 Billion by 2032, exhibiting a growth rate (CAGR) of 13.97% during the forecast period from 2024 to 2032.
Cardiac Prosthetic Devices Market: The global cardiac prosthetic devices market size reached US$ 6.7 Billion in 2023, and projected to reach US$ 12.3 Billion by 2032, exhibiting a growth rate (CAGR) of 6.8% during the forecast period from 2024 to 2032.
Foot and Mouth Disease (FMD) Vaccine Market: The global foot and mouth disease vaccine market size reached US$ 2,129.0 Million in 2023, and projected to reach US$ 3,861.8 Million by 2032, exhibiting a growth rate (CAGR) of 6.6% during the forecast period from 2024 to 2032.
Asthma Therapeutics Market: The global asthma therapeutics market size reached US$ 18 Billion in 2023, and projected to reach US$ 22.0 Billion by 2032, exhibiting a growth rate (CAGR) of 1.9% during the forecast period from 2024 to 2032.
Breath Analyzer Market; The global breath analyzer market size reached US$ 4.0 Billion in 2023, and projected to reach US$ 23.5 Billion by 2032, exhibiting a growth rate (CAGR) of 20.8% during the forecast period from 2024 to 2032.
Laser Hair Removal Market: The global laser hair removal market size is projected to exhibit a growth rate (CAGR) of 14.67% during the forecast period from 2024 to 2032.
Glioblastoma Multiforme Treatment Market: The global glioblastoma multiforme treatment market size reached US$ 2.1 Billion in 2023, and projected to reach US$ 3.9 Billion by 2032, exhibiting a growth rate (CAGR) of 6.7% during the forecast period from 2024 to 2032.
Drug Discovery Informatics Market: The global drug discovery informatics market size reached US$ 3.3 Billion in 2023, and projected to reach US$ 7.8 Billion by 2032, exhibiting a growth rate (CAGR) of 9.7% during the forecast period from 2024 to 2032.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800