Sanofi US
NEWS
Takeda hoped that its experimental therapy could become the first FDA-approved treatment for EoE. However, the FDA wants the company to initiate another clinical study with TAK-721.
Parkinson’s disease has been immensely challenging for biotech companies. A high rate of late-stage attrition in trials has led to a lack of approved therapies for the disease.
It’s not unusual for biopharma companies to end the year with a restructuring that includes job cuts. Here are some of these companies.
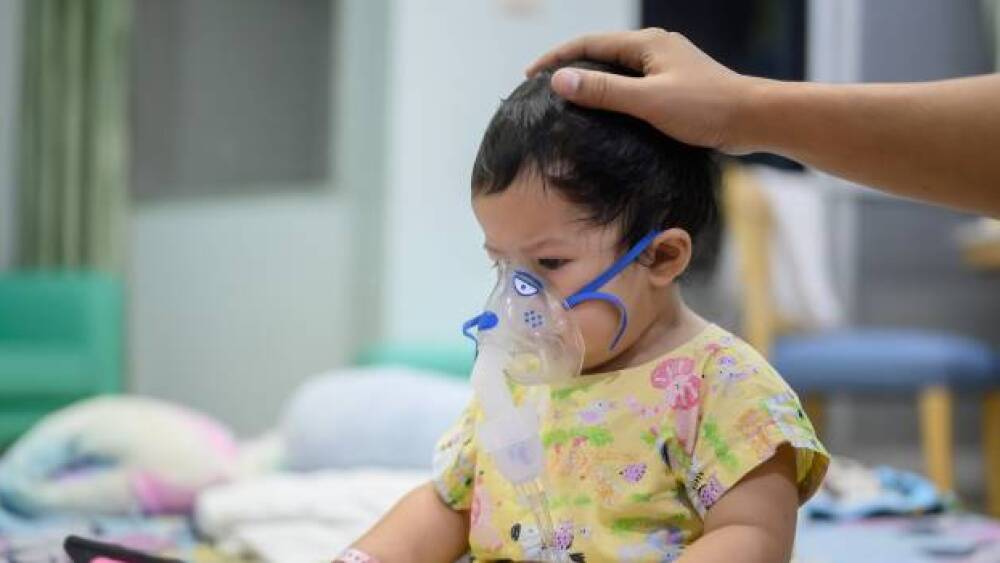
Respiratory Syncytial Virus has been around for a very long period now. But there was no vaccine yet. But, here’s the first vaccine for RSV to shape the future.
Researchers found improvements in the study’s co-primary endpoints in patient-reported measures of esophageal inflammation and difficulty swallowing.
Skin health logged major wins this week after two companies announced positive clinical trial results.
ReCode is aiming to advance its lead candidates for cystic fibrosis and primary ciliary dyskinesia (PCD).
Recently launched biotech company Immunai is pairing artificial intelligence and machine learning in an attempt to tailor treatments and improve patient outcomes.
Asher Bio began two years ago and raised $55 million in a Series A financing round this March to take forward a series of engineered cytokines that aim to eliminate both the toxicities and the loss of efficacy in immunotherapy.
JOBS
IN THE PRESS