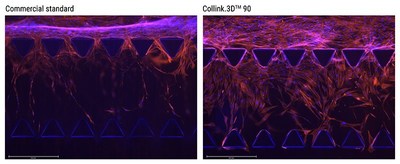
CollPlant today announced the Company is launching Collink.3D™ 90, a recombinant human collagen (rhCollagen)-based bioink for use in a variety of 3D bioprinting applications.
REHOVOT, Israel, Nov. 7, 2022 /PRNewswire/ -- CollPlant (Nasdaq: CLGN), a regenerative and aesthetics medicine company developing innovative human collagen-based technologies and products for tissue regeneration and organ manufacturing, today announced the Company is launching Collink.3D™ 90, a recombinant human collagen (rhCollagen)-based bioink for use in a variety of 3D bioprinting applications. Collink.3D™ 90 is complementary to the Company's first commercial bioink, Collink.3D™ 50, which was launched late last year, offering increased mechanical properties to address additional printing requirements of soft and hard tissues. Bioinks are applied to a diverse range of soft and hard tissue engineering applications, with each tissue or organ having specific mechanical requirements for fulfilling physiological needs. Thus, the most suitable bioink is required for a given application to mimic the physical properties of the target tissue or organ, while ensuring high cell survival. "We are very excited to continue to broaden our rhCollagen-based bioink offerings, providing our biopharma and academic customers the ability to print a variety of applications," said CollPlant CEO, Yehiel Tal. "We believe our bioinks deliver a favorable alternative to existing commercial bioinks owing to their high bio-functionality, rheological properties (e.g., controlled viscosity) and high purity. In addition to our product pipeline, bioinks are an important and strategic platform that supports CollPlant's efforts to pursue licensing and collaboration agreements with business partners, and we will continue to introduce new bioinks and innovate in this area", he added. Collink.3D™ 90 demonstrates faster human cell migration into gel matrices compared with a commercial hydrogel widely used for 2D and 3D cell culture. Cell migration plays a pivotal role in physiological processes such as tissue repair and regeneration, and these initial results indicate the potential for Collink.3D™ 90 to perform as a superior, animal free, human cell culture substrate. Collink.3D™ enables the high-resolution, scalable and reproduceable 3D bioprinting of scaffolds that accurately mimic the physical properties of human tissues and organs, with improved biofunctionality, safety and reproducibility. Applications for Collink.3D™ include its use in 3D cell culturing, tissue modeling for drug discovery and development, as well as engineering tissues and implantation of organs in regenerative medicine, representing a potential multi-billion-dollar market opportunity. Biofabricated constructs composed of Collink.3D™ offer superior biological performance, consistency and safety. Collink.3D™ is compatible with major 3D bioprinting technologies and cell types including stem cells, induced pluripotent stem cells, endothelial, and epithelial cells. More information can be found on https://collplant.com/products/collink3d/ Contact us for more details collink3d@collplant.com About CollPlant CollPlant is a regenerative and aesthetic medicine company focused on 3D bioprinting of tissues and organs, and medical aesthetics. The Company's products are based on its rhCollagen (recombinant human collagen) produced with CollPlant's proprietary plant based genetic engineering technology. These products address indications for the diverse fields of tissue repair, aesthetics, and organ manufacturing, and are ushering in a new era in regenerative and aesthetic medicine. In 2021 CollPlant entered into a development and global commercialization agreement for dermal and soft tissue fillers with Allergan, an AbbVie company, the global leader in the dermal filler market. For more information about CollPlant, visit http://www.collplant.com Safe Harbor Statements This press release may include forward-looking statements. Forward-looking statements may include, but are not limited to, statements relating to CollPlant's objectives plans and strategies, as well as statements, other than historical facts, that address activities, events or developments that CollPlant intends, expects, projects, believes or anticipates will or may occur in the future. These statements are often characterized by terminology such as "believes," "hopes," "may," "anticipates," "should," "intends," "plans," "will," "expects," "estimates," "projects," "positioned," "strategy" and similar expressions and are based on assumptions and assessments made in light of management's experience and perception of historical trends, current conditions, expected future developments and other factors believed to be appropriate. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements. Many factors could cause CollPlant's actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including, but not limited to, the following: the Company's history of significant losses, its ability to continue as a going concern, and its need to raise additional capital and its inability to obtain additional capital on acceptable terms, or at all; the impact of the COVID-19 pandemic; the Company's expectations regarding the timing and cost of commencing clinical trials with respect to tissues and organs which are based on its rhCollagen based BioInk and products for medical aesthetics; the Company's ability to obtain favorable pre-clinical and clinical trial results; regulatory action with respect to rhCollagen based BioInk and medical aesthetics products including but not limited to acceptance of an application for marketing authorization review and approval of such application, and, if approved, the scope of the approved indication and labeling; commercial success and market acceptance of the Company's rhCollagen based products in 3D Bioprinting and medical aesthetics; the Company's ability to establish sales and marketing capabilities or enter into agreements with third parties and its reliance on third party distributors and resellers; the Company's ability to establish and maintain strategic partnerships and other corporate collaborations; the Company's reliance on third parties to conduct some or all aspects of its product manufacturing; the scope of protection the Company is able to establish and maintain for intellectual property rights and the Company's ability to operate its business without infringing the intellectual property rights of others; the overall global economic environment; the impact of competition and new technologies; general market, political, and economic conditions in the countries in which the Company operates; projected capital expenditures and liquidity; changes in the Company's strategy; and litigation and regulatory proceedings. More detailed information about the risks and uncertainties affecting CollPlant is contained under the heading "Risk Factors" included in CollPlant's most recent annual report on Form 20-F filed with the SEC, and in other filings that CollPlant has made and may make with the SEC in the future. The forward-looking statements contained in this press release are made as of the date of this press release and reflect CollPlant's current views with respect to future events, and CollPlant does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Photo - https://mma.prnewswire.com/media/1939402/Collink_90_launch.jpg Contact at CollPlant:
SOURCE CollPlant |
||
Company Codes: NASDAQ-NMS:CLGN |