Clinical research
Armed with new late-stage data on reducing low-density lipoprotein cholesterol, Novartis is positioning its siRNA therapy Leqvio as a preventive treatment for atherosclerotic cardiovascular disease.
Neurocrine Biosciences’ potential competitor to Bristol Myers Squibb’s KarXT improved symptoms of schizophrenia in a Phase II trial, but only at the low dose tested.
The company announced Tuesday that despite the error preventing the analysis of late-stage results, the FDA confirmed that data from other completed trials are sufficient to support an NDA submission in the first quarter of 2025.
The number of patients who will be eligible for Novo Nordisk’s blockbuster GLP-1 under new Medicare Part D plan guidelines will vary depending on how cardiovascular disease is defined, according to researchers.
The retail pharmacy giant and the Biomedical Advanced Research and Development Authority hope to make decentralized clinical trials more accessible and representative of the U.S. population.
In June, the regulator placed a partial clinical hold on a Phase I trial of the companies’ antibody-drug conjugate after three patient deaths were reported.
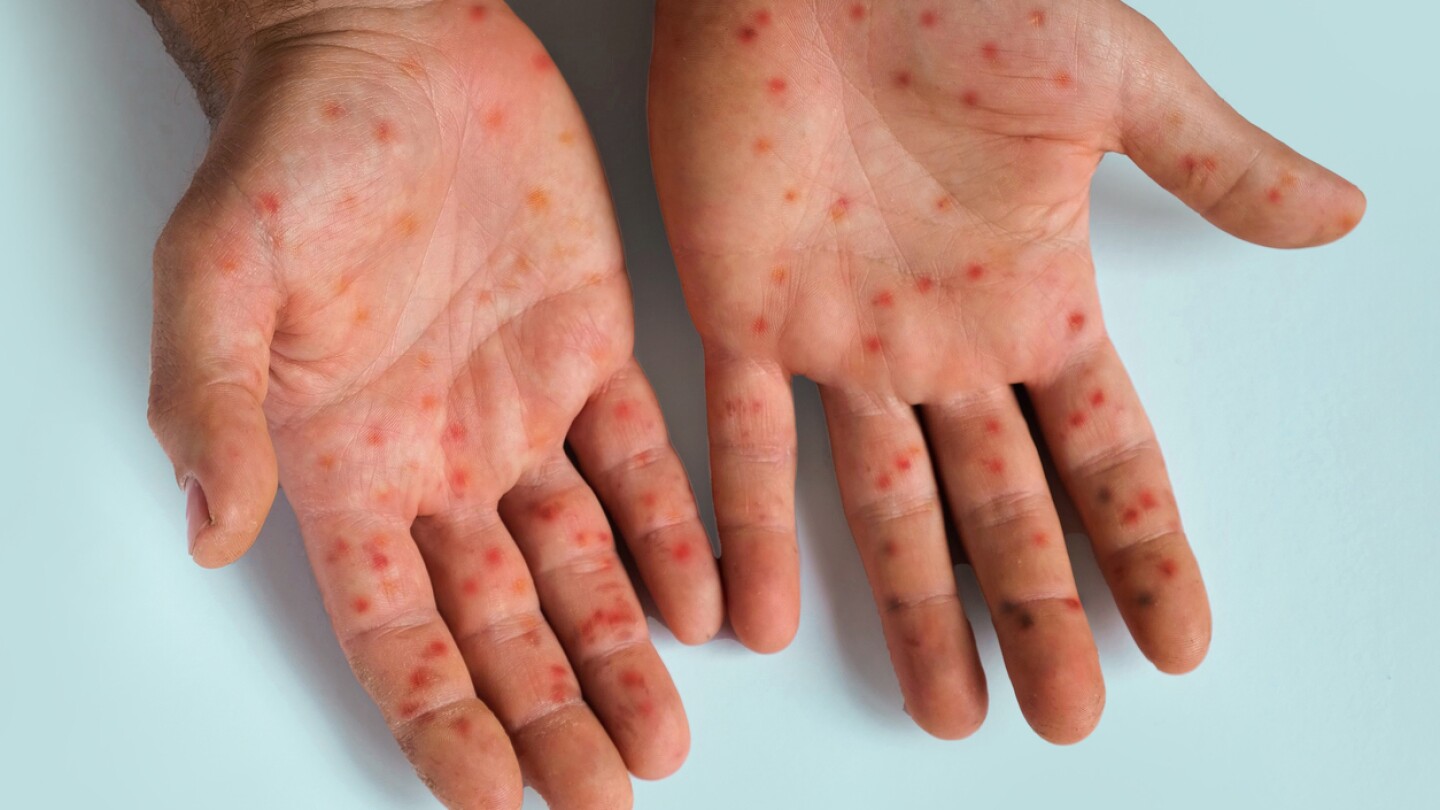
SIGA Technologies’ TPOXX did not outperform placebo at resolving lesions in patients with clade I mpox, the new strain that has spread through parts of Africa and is reaching beyond the continent.
The entry of new players and new approaches into the ATTR-CM space could help bring down the cost of treatment, experts say.
The companies’ late-stage stumble could allow Moderna to widen its lead, with its mRNA-based combination vaccine eliciting superior immune responses against COVID-19 and three influenza strains.
Lori and guests address clinical trial design, which if done without careful consideration of the patient population can exclude patients from clinical trials instead of being inclusive.
PRESS RELEASES