SHANGHAI, Oct. 20, 2016 /PRNewswire/ -- A team of scientists led by the iHuman Institute of ShanghaiTech University has determined and analyzed the high-resolution atomic structure of human cannabinoid receptor 1 (CB1), also known as the "marijuana receptor". This new findings provide clues to understand why some drugs that interact with this receptor have had unexpectedly complex and sometimes harmful effects, while the utility of the crystal structure may provide inspiration for drug design toward refining efficacy and avoiding adverse effects.
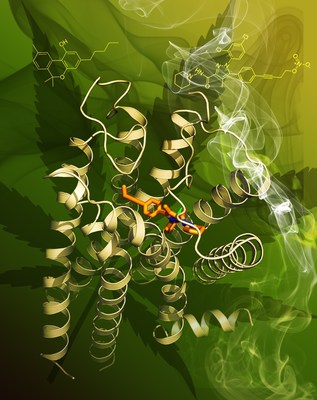
CB1 is one of the most highly expressed G protein-coupled receptors (GPCRs) in the human central nervous system and is the target of one of the longest used herbs in human history. Previous studies discovered that CB1 is the principal target of 9-tetrahydrocannabinol (THC), a psychoactive chemical from marijuana (Cannabis sativa) which has been used for therapeutic purposes for many centuries. CB1 is activated by endocannabinoids, and is a promising therapeutic target for pain management, inflammation, obesity, liver fibrosis and substance abuse disorders. However, CB1 has not been targeted to its full pharmaceutical potential and there is urgent need for achieving a better understanding of this receptor.
The laboratories of Professors Zhi-Jie Liu and Raymond Stevens at iHuman Institute have been working together to determine the 3-D atomic structures of cellular receptors -- particularly the large receptor family known as G protein-coupled receptors (GPCRs). GPCRs are responsible for more than 80% of signal transmission across cell membranes and are the targets of nearly 40% of today's marketed medicines. "The detailed molecular structure of the receptor in complex with agonist or antagonist will help people understand how cellular signaling is affected by drugs, and will offer a valuable framework for designing safer and more effective medicines", said iHuman Deputy Director and Professor Zhi-Jie Liu.
The first CB1-selective antagonist/inverse agonist, rimonabant (SR141716, Acomplia [Sanofi-Aventis]), received approval from the European Medical Agency (EMA) as an adjunct to diet and exercise for treating obesity. However, rimonabant and other ligands in its class were withdrawn from the EMA and were not approved in the United States due to concerns about adverse events, such as increased anxiety, depression, and suicidal ideation. Given the immense opportunities that CB1 provides as a therapeutic target, efforts have since focused on determination of the 3-D structure of CB1 with ligands to aid in the development of safer pharmacotherapies for overcoming such undesirable effects.
The Makriyannis lab at Northeastern University, USA teamed up with Liu-Stevens as part of the international effort on unveiling the 3D structure of CB1. The Makriyannis lab designed and synthesized AM6538, a strong stabilizing antagonist of CB1, specifically for structural studies. "The endocannabinoid field has needed this structural data for a long time, and we were thrilled to collaborate with the iHuman Institute to accomplish this great result", said Prof. Alex Makriyannis, Director and Founder of the Center for Drug Discovery.
The first author of this study, Tian Hua, a graduate student at Prof. Zhi-Jie Liu's lab, and her colleagues determined the crystal structure of CB1 in complex with AM6538 at 2.8 Å resolution. AM6538, synthesized by the second author Kiran Vemuri, is expected to have a long half-life and be potentially useful in the treatment of addiction disorders. Additionally, their studies found a number of structurally dissimilar CB1 ligands can be accommodated when docked into the binding domain defined by the CB1 crystal structure. This should allow a diverse set of CB1 ligands to interact with CB1 through different binding motifs and have distinct pharmacological profiles. iHuman Founding Director and Professor Raymond Stevens noted that, "With each new GPCR structure, we get closer and closer to understanding molecular recognition inside the human body. The CB1 receptor surprised us both with how incredibly hard it was to study, and also with the revelation of such a large and complex drug binding site. We look forward to following up on these studies and fully understanding how the receptor works."
Other co-authors of this paper are Yiran Wu, Suwen Zhao, Wenqing Shui, Shanshan Li of iHuman Institute at ShanghaiTech University, Mengchen Pu and Lu Qu of the Institute of Biophysics at Chinese Academy of Sciences, Kiran Vemuri, Nikolai Zvonok, Anisha Korde and Han Zhou of Northeastern University, USA, Gye Won Han of University of Southern California, Laura Bohn, Robert Laprairie, Edward Stahl and Jo-Hao Ho of The Scripps Research Institute, Florida, Irina Kufareva of University of California, San Diego, Beili Wu and Qiang Zhao of Shanghai Institute of Materia Medica, and Michael Hanson of the GPCR Consortium.
Funding support for these studies was provided by the Ministry of Science and Technology of China (grants 2014CB910400 and 2015CB910104), The National Nature Science Foundation of China (grant 31330019), National Institutes of Health (grants P01DA009158, R37DA023142 and R01AI118985), NSF grants, Shanghai Municipal Government, ShanghaiTech University and GPCR Consortium.
About the iHuman Institute (http://ihuman.shanghaitech.edu.cn) Located in the heart of Zhangjiang InnoPark in Shanghai, China, the Institute is the Asian gateway to the world for imaging across the scales. The iHuman Institute is focused on the basic and applied science of building an atomic resolution 3-dimensional model of the human body. By integrating molecular, cellular and organ imaging together, we can understand how all the parts work together to make us who we are. One of our dreams is to see routine and robust whole body imaging occur at the atomic resolution to detect diseases before it is too late to treat with medicines.
About ShanghaiTech University (http://www.shanghaitech.edu.cn/eng/). The university was jointly established by Shanghai Municipal Government and Chinese Academy of Sciences in 2013. Conveniently located in Zhangjiang InnoPark, Pudong, Shanghai, ShanghaiTech is in the vicinity of several national research institutes and large research facilities. ShanghaiTech is committed to serving the national development strategy and seeks innovative solutions to address the challenges that China is facing in the fields of energy, materials, environment, and human health, in order to improve innovation-driven productivity and contribute to the restructuring and development of China.
Xun Liu, PhD
Press Officer, ShanghaiTech University
Phone: +86-21-2068-5159
liuxun@shanghaitech.edu.cn

Photo - http://photos.prnewswire.com/prnh/20161019/430264
Photo - http://photos.prnewswire.com/prnh/20161019/430265
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/shanghaitech-scientists-resolve-the-structure-of-the-human-marijuana-receptor-300347438.html
SOURCE ShanghaiTech University





