Asia
Every week there are numerous scientific studies published. Here’s a look at some of the more interesting ones.
The research collaboration will see the development of bispecific antibodies and CAR-T cell products.
A busy week for clinical trial news as the ASCO meeting wrapped up and the European Hematology Association 2021 Virtual Congress began. Read on for more.
Beigene presented results from three pivotal trials at the 26th EHA2021 Virtual Congress for the effectiveness of their checkpoint inhibitor tislelizumab in relapsed or refractory lymphoma.
According to the CDC, the Delta variant now accounts for more than 6% of infections in the U.S. and may be responsible for 18% of cases in some Western states.
The Pennsylvania-based eye disease company had to change its application from an Emergency Use Authorization to a full BLA approval.
This week, multiple companies have partnered in attempts to bring forth new therapies. BioSpace takes a look at some of these announcements.
Biopharma and life sciences companies from across the globe provide updates on their businesses and pipelines.
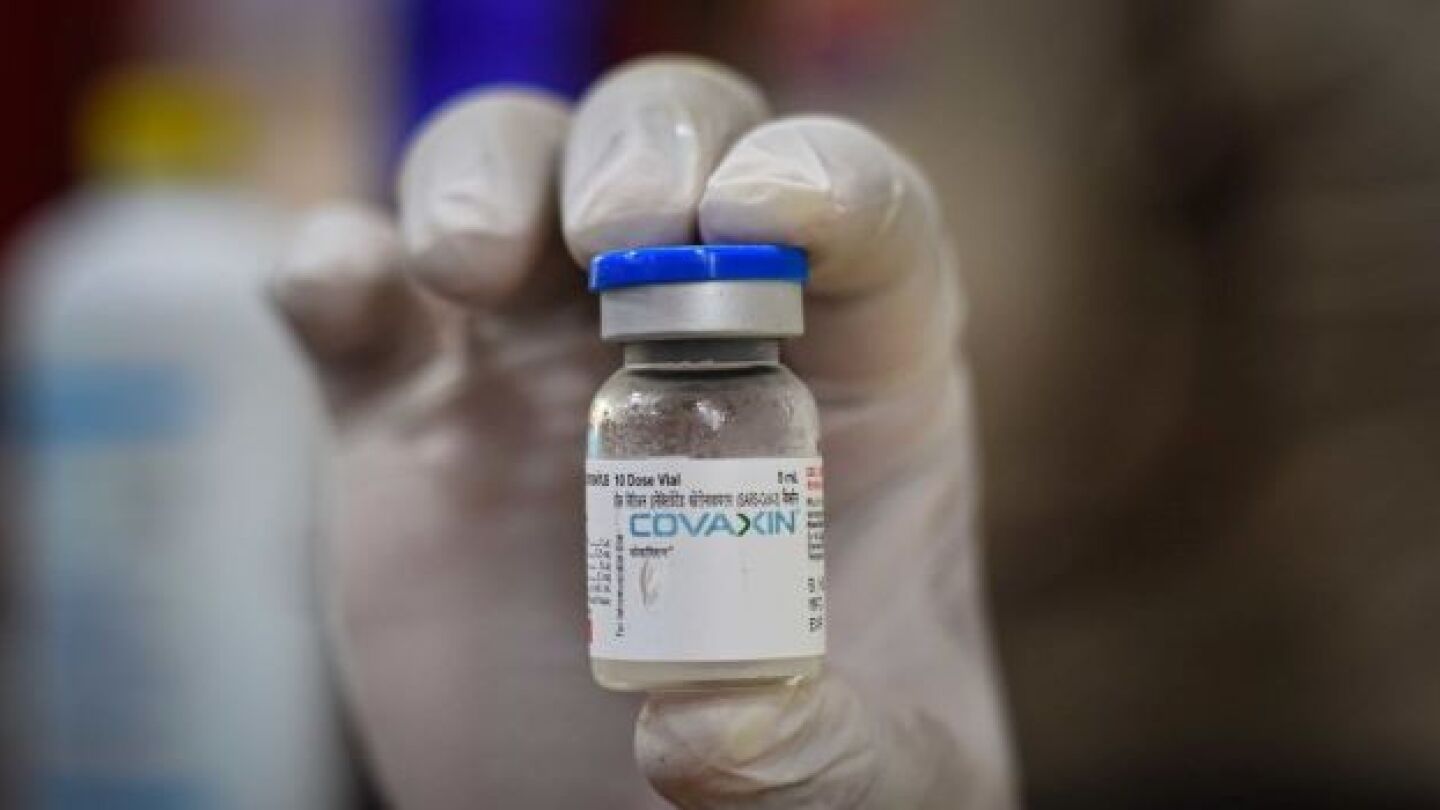
Bharat will conduct Phase IV studies to check real-world efficacy against the virus, while Ocugen says Covaxin will be a valuable tool in helping to end the COVID-19 pandemic.
Not everyone is so confident that Aduhelm will transform the Alzheimer’s drug development landscape.
PRESS RELEASES