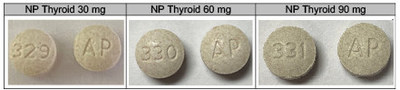
Acella Pharmaceuticals, LLC is voluntarily recalling a total of 13 lots of 30-mg, 60-mg and 90-mg NP Thyroid ® (thyroid tablets, USP) to the consumer level. The products are being recalled because our testing has found these lots to be superpotent. The product may have up to 115.0% of the labeled amount of Liothyronine (T3). Risk Stateme
|
ATLANTA, May 22, 2020 /PRNewswire/ --Acella Pharmaceuticals, LLC is voluntarily recalling a total of 13 lots of 30-mg, 60-mg and 90-mg NP Thyroid® (thyroid tablets, USP) to the consumer level. The products are being recalled because our testing has found these lots to be superpotent. The product may have up to 115.0% of the labeled amount of Liothyronine (T3). Risk Statement: Patients being treated for hypothyroidism (underactive thyroid), who receive superpotent NP Thyroid, may experience signs and symptoms of hyperthyroidism (overactive thyroid) which include, but are not limited to, weight loss, heat intolerance, fatigue, muscle weakness, hypertension, chest pain, rapid heart rate, or heart rhythm disturbances. Pregnant women who take superpotent NP Thyroid may also experience negative maternal and fetal outcomes including miscarriage and/or impairment to fetal development. Patients should talk to their healthcare professional before they stop taking their NP Thyroid medicine. To date, Acella has received two reports of adverse events known to be related to this recall. NP Thyroid (thyroid tablets, USP) is composed of levothyroxine and liothyronine, and used to treat hypothyroidism (underactive thyroid). The products subject to recall are packed in 100-count bottles. See product images. To best identify the product, the NDC's, Product Description, Lot Numbers and Expiration Dates are listed. These lots were distributed nationwide in the USA to Acella's direct accounts.
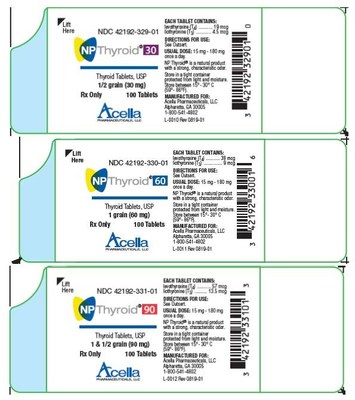
See Product Labels: Acella is proactively notifying its wholesalers by email and phone to discontinue distribution of the product being recalled and is arranging for return of all recalled products. Patients who are currently taking NP Thyroid from the lots being recalled should not discontinue use without contacting their healthcare provider for further guidance and/or a replacement prescription. Consumers with questions about the recall can email Acella Pharmaceuticals at recall@acellapharma.com or contact Acella Customer Service at 1-800-541-4802, Monday through Thursday from 9:00 am to 5:00 pm ET and Friday from 9:00 am to 12:30 pm ET. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product. Adverse events or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
SOURCE Acella Pharmaceuticals, LLC. |