Theralase® Technologies Inc. is pleased to announce that in preclinical research, it’s lead drug formulation, Rutherrin® has been proven effective in increasing the efficacy of chemotherapy and reducing multidrug resistance.
TORONTO, ON / ACCESSWIRE / June 12, 2024 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation activated small molecules and their formulations intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that in preclinical research, it's lead drug formulation, Rutherrin® has been proven effective in increasing the efficacy of chemotherapy and reducing multidrug resistance.
Chemotherapy is currently one of the principal treatment methods for cancer, along with radiation and surgery. Clinically, many tumours undergo a satisfactory response, when first exposed to chemotherapeutic drugs; however, despite the initial success of these treatments, growing resistance to treatment with these drugs becomes a common occurrence. This results in the steady loss of therapeutic response over time for cancer patients, despite the wide spectrum of drugs and treatments available. This phenomenon is termed Multi-Drug Resistance ("MDR").
Although there are several different mechanisms associated with the development of MDR in patients, a common cause is believed to be the overexpression of a plasma membrane superfamily of transporter proteins, called the ATP-Binding Cassette ("ABC") transporter, which act as an energy-dependent drug efflux pump, preventing adequate intracellular accumulation of a broad range of cytotoxic drugs. In other words, the chemotherapeutic drugs are expelled by the cancer cells, before they have a chance to be effective. This underlines the critical importance of identifying compounds that could inhibit the efflux pump activities.
In order to determine the effect of Rutherrin® on MDR in cancer cells, cells were treated with Rutherrin®, before addition of one of the following drugs:
- Hoechst 33342 (nuclear dye commonly used to study ABC transporter drug efflux)
- Temozolomide (chemotherapy used to treat brain cancer)
- Gemcitabine (chemotherapy used to treat various cancers)
- Cisplatin (platinum-based chemotherapy used to treat various cancers)
Following incubation, cells were washed and the amount of intracellular drug was quantified using High-Performance Liquid Chromatography coupled with Mass Spectrometry.
The amount of drug was normalized to cells, which were not treated with Rutherrin®, as a control.
As shown in Figure 1, treatment with Rutherrin® significantly enhanced the retention of all tested chemotherapeutic drugs, presumably through the inhibition of the ABC transporter efflux pump, resulting in higher intracellular drug accumulation, which would increase exposure of the cancer cells to the respective chemotherapy and consequently improve overall treatment efficacy.
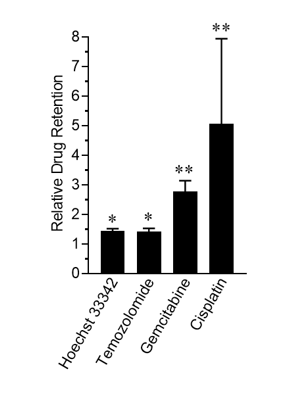
Figure 1: Relative chemotherapeutic drug retention in Rutherrin® treated cells normalized to drug-only treated cells
To further investigate this phenomenon and to demonstrate cancer cell kill, in vitro cells were treated with Rutherrin®, before addition of various chemotherapeutic drugs to analyze cell survival.
These chemotherapeutic drugs included:
- Vandetanib (chemotherapy used to treat thyroid cancer)
- Vemurafenib (chemotherapy used to treat melanoma)
- Vinblatine (chemotherapy used to treat lymphoma, breast cancer and testicular cancer)
- Cisplatin (platinum-based chemotherapy used to treat various cancers)
- Temozolomide (chemotherapy used to treat brain cancer)
- Gemcitabine (chemotherapy used to treat various cancers)
As shown in Figure 2, addition of Rutherrin® significantly increased the cancer cell kill for all tested chemotherapeutic drugs, suggesting a universal effect of Rutherrin® on chemotherapeutic drugs in their destruction of cancer cells, rendering the cancer cells more susceptible to chemotherapy.
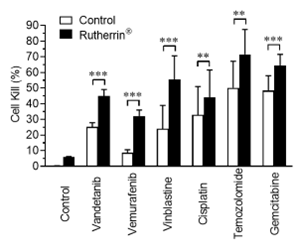
Figure 2: Cell kill percent after treatment with chemotherapeutic drugs (listed on X-axis) (+/- Rutherrin® treatment)
To further validate the research, a mouse animal model was utilized, where the mice were injected subcutaneously with mouse colorectal cancer cells and divided into four treatment groups; specifically: Untreated Control, Rutherrin® only, Vinblastine only and Rutherrin® combined with Vinblastine.
As shown in Figure 3, the combination of Rutherrin® and Vinblastine significantly delayed tumor volume progression and enhanced overall animal survival, compared to control or either treatment alone. This research was completed using Rutherrin® with no light or radiational activation.
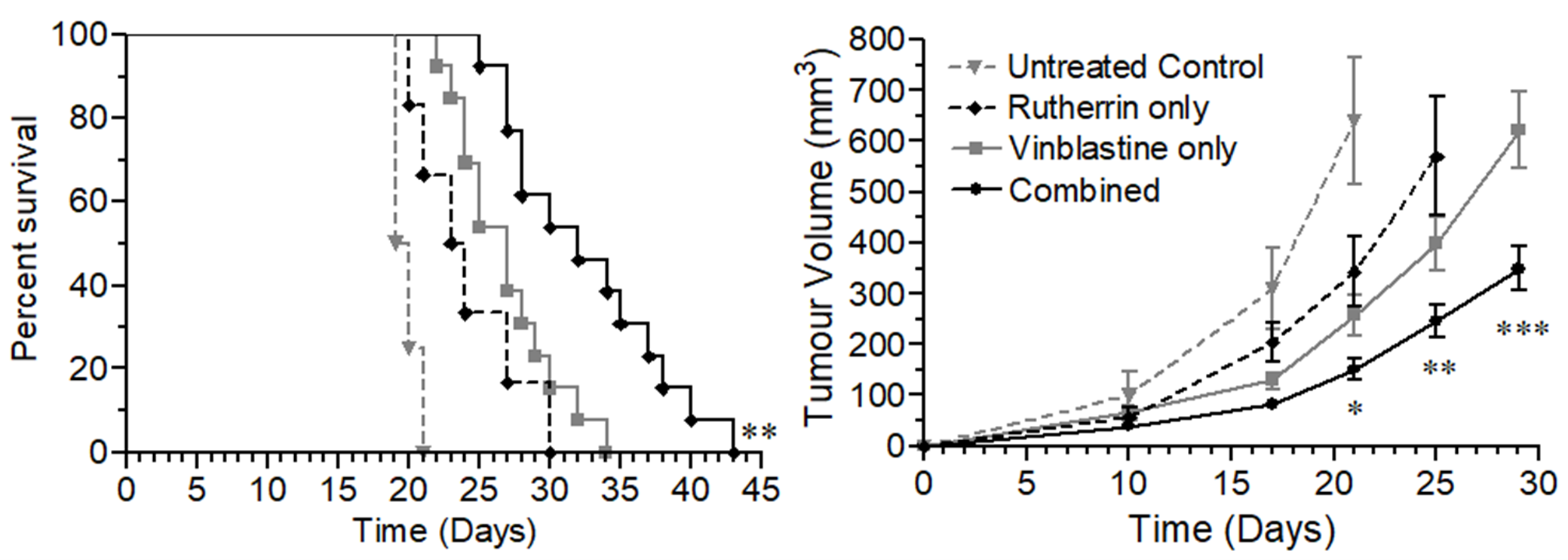
Figure 3: Kaplan-Meier survival curve and tumor volume analysis in mice after subcutaneous tumour inoculation and treatment with either: Control, Rutherrin® only, Vinblastine only or combined Rutherrin®, plus Vinblastine treatment
Dr. Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase® stated," Nearly all current cancer treatments face a common issue, the chemotherapy used initially is effective, but becomes less and less effective over time, creating a phenomenon known as Multi-Drug Resistance ("MDR"). MDR is one of the most challenging problems facing cancer researchers, clinicians and patients today. In order to overcome MDR, this research strongly suggests that the effectiveness of chemotherapy over time could a minimum be maintained or even significantly enhanced through the addition of Rutherrin® to mainstream cancer care. Our methodology is not to treat patients with combinations of different drugs, as research has shown that the systemic delivery of multiple drugs can cause unintended adverse effects, drug to drug interactions and may even diminish the overall anti-cancer efficacy and patient compliance. Therefore, a more effective alternative would be to combine Rutherrin® with chemotherapy to increase the overall treatment efficacy by modulating the "drug efflux", one of the major hallmarks of tumor resistance. The "drug efflux" is essential for tumor cell proliferation and migration, thus playing a pivotal role in tumor physiology. Treatment of various cancer cells with Rutherrin® allows chemotherapeutic drugs to remain in cancer cells in higher quantities and for longer times. In addition, this tumor-specific accumulation of anti-cancer drugs can help prevent the unnecessary exposure of healthy cells to chemotherapy; thus, increasing treatment efficacy and minimizing the adverse effects of anti-cancer therapy. We are very pleased with the results of our latest experiments, which have paved the way for a clinical study investigation of Rutherrin®, combined with chemotherapy, for synergistic combinational cancer treatment to overcome MDR."
Roger DuMoulin-White, B.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase® stated, "We are delighted with the recent preclinical research confirming that our patented, metal-based, small molecule, Rutherrin® formulation can effectively combat MDR, as well as increase the efficacy of chemotherapy. Clinically, MDR to chemotherapy, targeted therapy or immunotherapy remains the greatest impediment towards curative and long-lasting cancer therapy. This leads directly to treatment failure, high patient morbidity and mortality, as well as increased medical costs through extensive repeat medical treatments and extended hospital stays. In this respect, Theralase®'s small molecule formulations possess significant potential in optimizing cancer treatments, as it is well documented scientifically, that the transferrin receptor, which is the target of Rutherrin®, is highly expressed in virtually all cancer cell types."
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains "forward-looking statements" within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. Forward looking statements may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of Company's management for future research, development and commercialization of the Company's small molecules and their drug formulations, preclinical research, clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations, the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or at all, the risk that the Company's small molecule and drug formulations may not be effective against the diseases tested in its clinical studies, the risk that the Company's fails to comply with the term of license agreements with third parties and as a result loses the right to use key intellectual property in its business, the Company's ability to protect its intellectual property, the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these forward-looking statements, which are not a guarantee of future performance. There can be no assurance that forward-looking statements will prove to be accurate as such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the forward-looking statements.
Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements.
All forward-looking statements are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
X 224
khachey@theralase.com
SOURCE: Theralase Technologies Inc.
View the original press release on accesswire.com
