Regulatory
GenAI, the youngest of the AI family, is evolving quickly. The global life sciences industry is attempting to evolve with it. However, as a keynote session at DIA’s MASC suggests, taking a pause to understand AI risks and to prepare to comply with the EU AI Act is what is needed.
Orchard Therapeutics on Monday secured the FDA’s first approval for an autologous gene therapy to treat the rare metabolic disease metachromatic leukodystrophy in children.
By votes of 11-0 and 8-3, respectively, an FDA advisory committee Friday deemed the risks of early death for both Johnson & Johnson’s Carvykti and Bristol Myers Squibb’s Abecma acceptable.
The FDA approved Bristol Myers Squibb’s Breyanzi for chronic lymphocytic leukemia and small lymphocytic leukemia prior to Friday’s adcomm for the company’s other CAR-T therapy, Abecma.
Despite skepticism from FDA reviewers, the Oncologic Drugs Advisory Committee on Thursday strongly supported Geron’s imetelstat for the treatment of anemia in patients with lower-risk myelodysplastic syndromes.
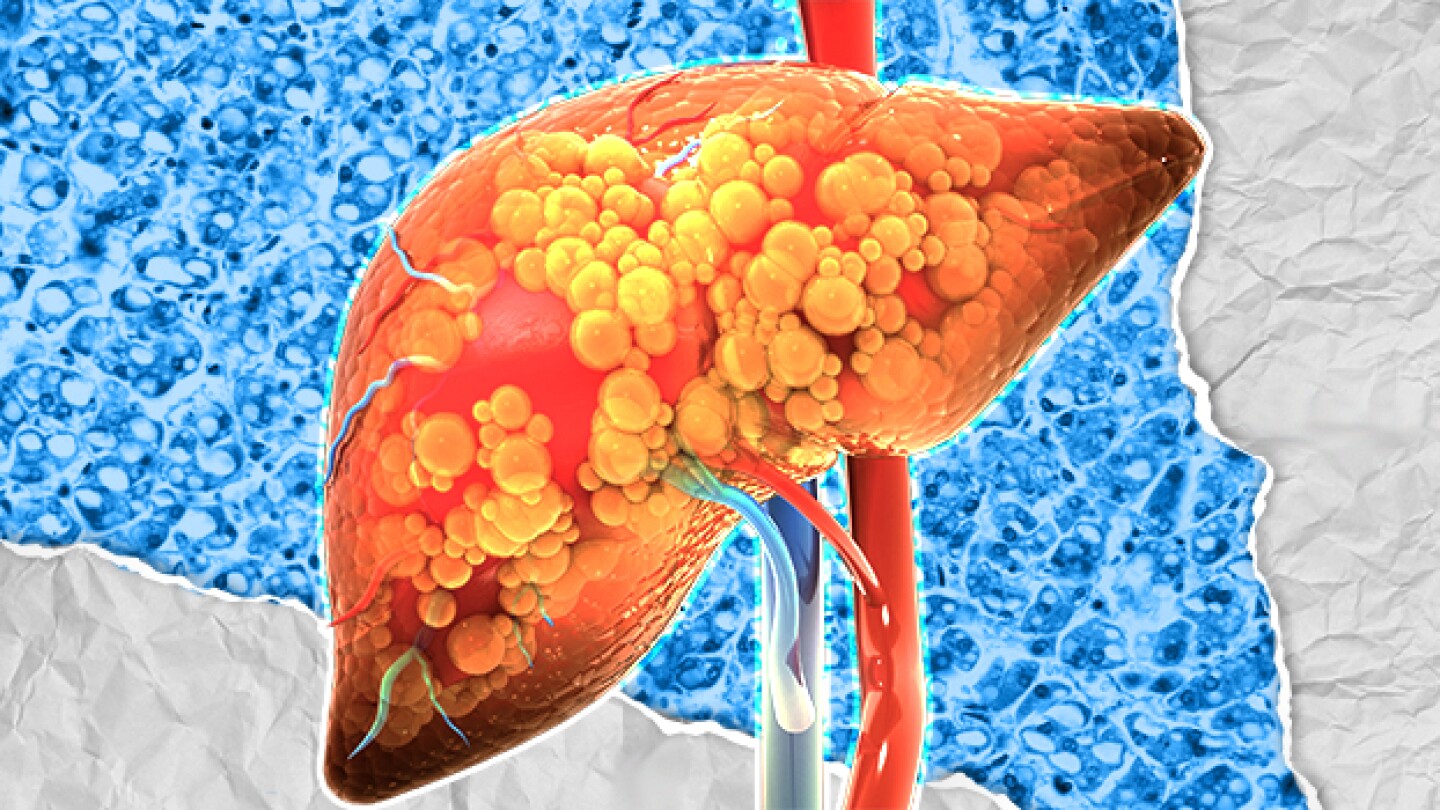
Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) is the first-ever approved therapy for metabolic dysfunction-associated steatohepatitis—a decision experts say could signal a sea change in treatment of the disease.
The U.K. National Institute for Health and Care Excellence on Thursday recommended against funding Vertex Pharmaceuticals’ CRISPR-based sickle cell disease therapy Casgevy unless uncertainties can be cleared up.
With an advisory committee meeting slated for Friday, the regulator has posted briefing documents in which it has raised concerns about early deaths in patients treated with Bristol Myers Squibb’s Abecma and Johnson & Johnson’s Carvykti.
The FDA will close out a hectic month of March with a flurry of target action dates, including ones for lymphoma and CKD anemia treatments.
In a briefing document for Thursday’s advisory committee meeting, the FDA pointed to efficacy and safety issues with Geron’s New Drug Application for imetelstat in myelodysplastic syndromes.
PRESS RELEASES