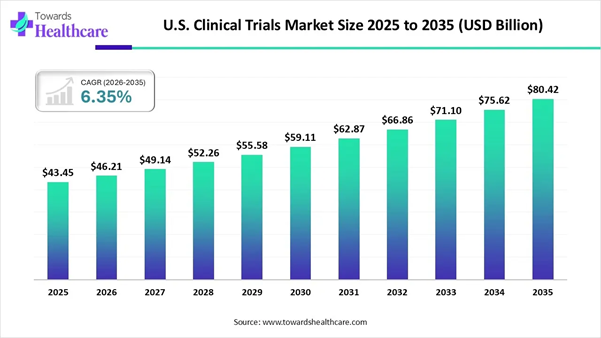
According to Towards Healthcare market projections, the U.S. clinical trials market is expected to grow from USD 43.45 billion in 2025 to USD 80.42 billion by 2035, reflecting a CAGR of 6.35%, due to rising government investment and rising collaboration among key players.
The market is growing due to rising investment in novel therapies, precision medicine, and advanced biotech innovations. Increasing disease prevalence and a supportive regulatory framework are also accelerating trial demand and expansion.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/6455
Key Takeaways
➤ The U.S. clinical trials market will likely exceed USD 43.45 billion by 2025.
Valuation is projected to hit USD 80.42 billion by 2035.
➤ Estimated to grow at a CAGR of 6.35% starting from 2026 to 2035.
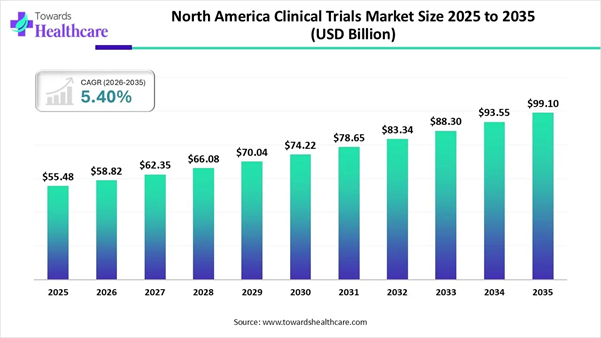
➤ The North America clinical trials market is US$ 55.48 billion in 2025, projected to reach US$ 99.10 billion by 2035 at a 5.40% CAGR.
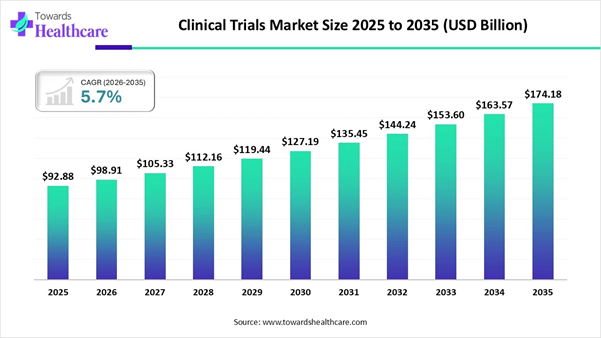
➤ The clinical trials market will grow from USD 54.39 billion in 2024 to USD 94.68 billion by 2034, at a 5.7% CAGR.
➤ By phase, the phase 3 segment dominated the U.S. clinical trials market in 2024.
➤ By phase, the phase 2 segment is expected to grow at the fastest CAGR during 2025-2034.
➤ By study design, the interventional study segment dominated the market in 2024 and is expected to grow at the fastest CAGR during 2025-2035.
➤ By intervention, the oncology segment dominated the market in 2024 and is expected to grow the fastest CAGR during 2025-2034
What are U.S. Clinical Trials?
Clinical trials are structured research studies conducted on humans to evaluate the safety, effectiveness, and optimal use of new drugs, medical devices, or treatments. The U.S. clinical trials market is expanding due to strong R&D investments, rapid growth in biotechnology and precision medicine, and rising demand for innovative therapies across oncology, rare diseases, and chronic conditions. The country benefits from advanced healthcare infrastructure, a large patient pool, and access to skilled researchers. Supportive FDA initiatives, faster approval pathways, and widespread adoption of digital and decentralized trial technologies further accelerate trial execution, improving efficiency and boosting overall market growth.
What are the Core Drivers shaping the Rapid Development of the U.S. Clinical Trials Market?
The major drivers of the market include strong R&D investment, growing biotech and precision medicine innovation, and increasing demand for advanced therapies. The country’s robust healthcare infrastructure, diverse patient pool, and experienced research workforce boost trial efficiency. FDA support, faster approval routes, and adoption of digital and decentralized trial models further accelerate market expansion.
What are the Key trends in the U.S. Clinical Trials Market in 2024?
➤ In July 2024, Charles River Laboratories International, Inc. partnered with the FOXG1 Research Foundation to speed up the development of potential treatments for FOXG1 syndrome. The collaboration focuses on supporting and advancing drug candidates into the clinical trial stage more efficiently.
➤ In March 2024, Thermo Fisher Scientific Inc. introduced a CorEvitas clinical registry focused on Generalized Pustular Psoriasis (GPP) to strengthen real-world evidence. The registry collects detailed patient data to better understand disease progression, treatment use, and associated health conditions.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What is the Appearing Challenge in the U.S. Clinical Trials Market?
A key challenge in the U.S. clinical trials market is patient recruitment and retention, as many studies struggle to enroll a diverse and adequate population on time. Rising trial complexity, high operational costs, and strict regulatory further slow progress. Additionally, data management burdens and limited access to underserved communities create barriers that impact trial effectiveness and outcomes.
Segmental Insights
By Insights
How did the Phase 3 Segment Dominate the U.S. Clinical Trials Market in 2024?
The phase 3 segment held the largest market share in 2024 because this stage involves large-scale patient populations, extensive clinical evaluation, and comprehensive safety and efficacy assessment, making it the most resource-intensive part of drug development. Pharmaceutical companies invest heavily in phase 3 to generate the robust data required for regulatory approvals. These trials also have higher operational costs, longer durations, and greater complexity, which collectively contribute to their dominant market share.
The phase 2 segment is expected to grow at the fastest CAGR in the U.S. clinical trials market because it focuses on evaluating treatment efficacy and optional dosing, attracting strong investment from pharma and biotech companies developing targeted and precision therapies. Rising demand for early-stage data, growth in rare diseases research, and an increasing number of innovative drug candidates entering mid-stage support this expansion. Additionally, faster timelines and lower costs compared to phase drive greater activity in this phase.
By Study Design Insights
What made the Interventional Study Segment Dominant in the U.S. Clinical Trials Market in 2024?
The interventional study segment dominated the market and is projected to grow at the fastest CAGR because interventional studies are essential for testing the safety and effectiveness of new drugs, devices, and therapies. With rising investment in innovative treatment in innovative treatments, precision medicine, and targeted therapies, sponsors rely heavily on interventional-based trials to generate strong clinical evidence. Their structured design, regulatory importance, and ability to demonstrate clear treatment outcomes drive both higher demand and continued rapid growth throughout the forecast period.
By Intervention Insights
Why the Oncology Segment Dominated the U.S. Clinical Trials Market in 2024?
The oncology segment held the highest market share in 2024 and is expected to grow at the fastest CAGR due to rising global cancer burden and strong demand for advanced, targeted, and immunotherapy-based treatments. Pharma and biotech companies are heavily investing in innovative cancer drugs, driving a high volume of clinical trials. Additionally, supportive regulatory pathways, technological advances in biomarkers, and personalized medicine approaches continue to accelerate oncology trials activity and market growth.
➤ According to the U.S. FDA and multiple industry reports, pharmaceutical companies are now putting more than USD 38.0 billion into the pre-clinical and clinical development of cancer therapies. This investment reflects the strong push to advance new and effective oncology treatments.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
What Factors Support the U.S. Clinical Trials Market’s Growth?
The U.S. clinical trials market is growing rapidly due to the rising prevalence of clinical trials. Involvement of a government organization and efforts taken by academic and research institutions. Apart from this, the U.S. is in a dominant position in pharmaceuticals, biotechnology, and other life sciences sectors, which are heavily involved in clinical trials. Furthermore, the strong presence of major players competing in the region to develop novel, advanced therapeutics to stand out in the market and expand their global presence.
Clinical Trials Statistics in the U.S.
➤ Statistics for 2024: During the year, 43,674 new studies were posted on ClinicalTrials.gov Trends and Charts, with oncology being the most popular category.
➤ 2025 statistics: Over 36,700 studies have been published so far this year, indicating a robust recovery in research activity. By mid-2025, phase I–III trial starts had increased by 20% year over year.
North America Clinical Trials Market Growth
The North America clinical trials market size is calculated at US$ 55.48 billion in 2025, grew to US$ 58.82 billion in 2026, and is projected to reach around US$ 99.10 billion by 2035. The market is expanding at a CAGR of 5.40% between 2026 and 2035.
Global Clinical Trials Market Growth
The global clinical trials market size is calculated at USD 54.39 billion in 2024 and is expected to be worth USD 94.68 billion by 2034, expanding at a CAGR of 5.7% from 2024 to 2034, as a result of growing demand for personalized medicines, rising investment in R&D and adoption of decentralized clinical trials.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Recent Developments in the U.S. Clinical Trials Market
➤ In November 2025, the American Cancer Society expanded its ACS ACTS program nationwide to enhance equitable access to clinical trials. The service helps patients, caregivers, and clinicians find personalized trial options and provides trained specialists who guide users through trial understanding and overcome participation barriers.
➤ In August 2025, the U.S. Department of Defense awarded $7.3 million to researchers from VCU Massey Comprehensive Cancer Center and MD Anderson Cancer Center. The funding supports a new clinical trial focused on metastatic triple-negative breast cancer, one of the most difficult and aggressive breast cancer types to treat.
U.S. Clinical Trials Market Key Players List
▸ IQVIA
▸ Fortrea Inc.
▸ PAREXEL International Corporation
▸ Thermo Fisher Scientific Inc.
▸ Charles River Laboratories
▸ ICON Plc
▸ Wuxi AppTec Inc.
▸ Medpace
▸ Syneos Health
▸ AstraZeneca
▸ Merck & Co.
▸ Eli Lilly and Company
▸ Novo Nordisk A/S
▸ Pfizer
▸ Caidya
Segment Covered in the report
By Phase
• Phase 1
• Phase 2
• Phase 3
• Phase 4
By Study Design
• Observational Study
• Interventional Study
• Expanded Access Study
By Indication
• Autoimmune/Inflammation
○ Rheumatoid arthritis
○ Multiple Sclerosis
○ Osteoarthritis
○ Irritable Bowel Syndrome (IBS)
○ Others
• Pain Management
○ Chronic Pain
○ Acute Pain
○ Oncology
○ Blood Cancer
○ Solid Tumors
○ Other
• CNS Condition
○ Epilepsy
○ Parkinson's Disease (PD)
○ Huntington's Disease
○ Stroke
○ Traumatic Brain Injury (TBI)
○ Amyotrophic Lateral Sclerosis (ALS)
○ Muscle Regeneration
○ Others
• Diabetes
○ Obesity
○ Cardiovascular
○ Others
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/6455
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

