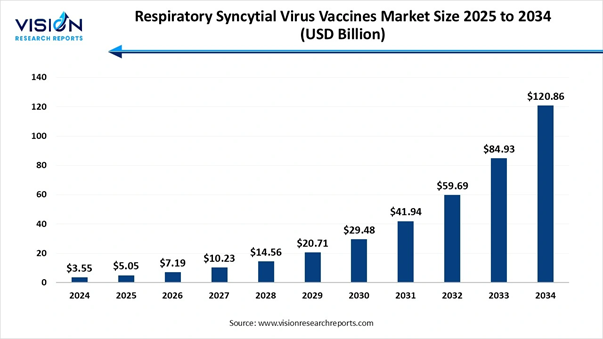
The global respiratory syncytial virus vaccines market size was calculated at USD 3.55 billion in 2024 and is expected to grow steadily from USD 5.05 billion in 2025 to reach around USD 120.86 billion by 2034, growing at an impressive CAGR of 42.3% from 2025 to 2034, Study Published by Vision Research Report.
The demand for this vaccine is due to the main health load that the virus carries to vulnerable populations, which includes older adults and infants, too. RSV can cause major respiratory illness that leads to death and hospitalization, specifically in the elderly and young infants.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Preview the Report Before You Buy – Get Sample Pages 👉 https://www.visionresearchreports.com/report/sample/41757
What is the Respiratory Syncytial Virus Vaccines Market?
Respiratory vaccines can protect against diseases caused by infection with the respiratory syncytial virus (RSV), as this disease points to an infection of the lower respiratory tract, which is caused by RSV. It is a prevalent virus that causes mild and cold-like symptoms. Hence, RSV is a serious disease that can be more damaging in older adults and infants.
RSV is generally transmitted through direct contact with the virus, such as droplets from another person’s sneeze or cough that contact your eyes, mouth, or nose. It can also be circulated by touching a surface that has the virus on it, such as a doorknob, and then touching any face before washing hands.
The RSV vaccine assists in protecting against the virus that causes the RSV disease. There are several types of RSV vaccines. The classifications between the vaccines are dependent on how they are created and which individuals should receive them.
Respiratory Syncytial Virus Vaccines Market Key Highlights:
• By region Asia Pacific region led with largest market share of 31% in 2024.
• By type, the passive immunization is segment contributed the largest market share of 76% in 2024.
• By type, the preventive vaccines segment is predicted to grow at the remarkable CAGR of 49.53% from 2025 to 2034.
• By technology, the monoclonal antibodies segment captured the maximum market share in 2024.
• By technology, the recombinant protein combined with adjuvant segment is expected to expand at the highest CAGR from 2025 to 2034.
• By targeted population, the Infants and children segment generated the maximum market share of 76% in 2024.
• By targeted population, the adult segment is estimated to expand the fastest CAGR during the forecast period.
• By distribution channel, the hospital and retail pharmacies segment registered the maximum market share of 54% in 2024.
• By distribution channel, the government suppliers segment is expected to grow at the notable CAGR from 2025 to 2034.
Latest Trends in the Respiratory Syncytial Virus Vaccines Market
Depending on the current situation and current developments, the trends in the respiratory syncytial virus (RSV) vaccines rely on a stretching scenario of existing products for the older adults, as well as the successful usage of a maternal vaccine in order to protect infants, and the constant growth of new technologies. Actual data is confirming the smoothness of checked vaccines while the policymakers update the suggestions for focused immunization.
The developers are discovering live-attenuated, nanoparticle-based vaccine candidates and the recombinant vector, too. Efforts are in progress to make an integration of vaccines that can focus on both RSV and other respiratory viruses like influenza.
Discover the Full Market Insights 👉
https://www.visionresearchreports.com/respiratory-syncytial-virus-vaccines-market/41757
What is the Opportunity for the Respiratory Syncytial Virus Vaccines Market?
In the last few years, there have been many successful trials that have been published for vaccines and monoclonal antibodies to prevent RSV disease. A bivalent perfusion F vaccine (RSVpreF) was seen to be effective against medically attended severe RSV-linked LRTI in infants and is now suggested by the CDC for pregnant women at 32-36 weeks.
Nirsevimab, which is a monoclonal antibody, was seen to be effective in protecting against major RSV-associated LRTi and connected hospitalisation when given to infants who are less than 12 months of age.
What are the Limitations for the Respiratory Syncytial Virus Vaccines Market?
In order to implement RSV vaccines, the globe has seen the effect of RSV-linked paediatric admissions to intensive care, community-dependent death, and complicated disease in LMICs, which need to be additionally measured, specifically linking to the neonatal period, in which data are being distributed. The vaccine probe research can use the effect of current developments in RSV protection procedure, which includes LRTI, mortality, inaccurate usage, chronic lung complexities, transmission, and the microbiome of the respiratory tract.
Technological Advancements in Respiratory Syncytial Virus Vaccines Market
Investigators at the Helmholtz Centre for Infection Research (HZI) have developed the latest vaccine technology. Their research to date displays just one dose of vaccine that leads to productive and long-lasting immune protection. The foundation of this so-called MCMV vaccine vector technology is called mouse cytomegalovirus (MCMV). It officially behaves as a carrier virus that reveals chosen antigens of the pathogen, which need to be vaccinated against in the body.
Government Support for Respiratory Syncytial Virus Vaccines Market
• In March 2025, the World Health Organization (WHO) prequalified the primary maternal respiratory syncytial virus (RSV) vaccine in order to protect infants against one of the most prevalent causes of severe lower respiratory infections in children worldwide.
• The Australian Immunisation Handbook has suggested the RS vaccination for the particular groups, which include pregnant women at 28 to 36 weeks of pregnancy.
• The people who are aged 75 years and older are the Aboriginal and Torres Strait Islander people, who are also the people who are aged 60 years and older.
• Also, the fold with the medicinal risk factors for many RSV diseases is aged 60 years and older.
• In July 2025, officially, three RSV vaccines are being licensed by the U.S. Food and Drug Administration for use in adults of various ages in the United States, which are known as Moderna’s mResvia, GSK’s Arexvy, and Pfizer's Abrysvo.
Respiratory Syncytial Virus Vaccines Market Report Coverage
|
Report Attribute |
Key Statistics |
|
Market Size in 2025 |
USD 5.05 Billion |
|
Market Size in 2026 |
USD 7.19 Billion |
|
Market Size in 2030 |
USD 29.48 Billion |
|
Market Size in 2032 |
USD 59.69 Billion |
|
Market Size by 2034 |
USD 120.86 Billion |
|
Growth rate from 2025 to 2034 |
CAGR of 42.3% |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2034 |
|
Segments Covered |
By Type, By Technology, By Targeted Population, By Distribution Channel |
|
Companies Covered |
GSK plc, Pfizer Inc., AstraZeneca plc, Moderna, Inc., Sanofi S.A., Bavarian Nordic A/S, Novavax, Inc., Johnson & Johnson (Janssen Pharmaceuticals), Merck & Co., Inc., Meissa Vaccines, Inc. |
For Orders or Inquiries, Don’t Hesitate to Reach Out: sales@visionresearchreports.com
Respiratory Syncytial Virus Vaccines Market Regional Analysis
The North America dominated the respiratory syncytial virus vaccines market share 31% in 2024, as the demand for this vaccine in the North America region displays a fast expansion since 2023, with the latest checked vaccines for the older adults, pregnant women, and infants too, which penetrate the industry. Despite this development, the starting uptake has been lower, and the main disparities in permission continue. In June 2024, the CDC upgraded its rule to suggest the vaccine for every adult who is 75 and older, too, and for those aged between 60 and 74 with particular risk factors. These risk-dependent and clearer suggestions are predicted to develop the future coverage.
United States Respiratory Syncytial Virus Vaccines Market Trends
The growth for the RSV vaccines industry in the United States feature both main development and space for improvement. While the vaccines' smoothness in the actual world is proving to be strong, less than desired uptake, logistical obstacles show the demand for constant public health efforts and resolute demographic and socioeconomic disparities, too. The Educational campaigns that solve the vaccine hesitancy and address the severe procedure will be key to developing equal access and maximizing the overall public health effect of these new protective tools.
The Asia Pacific region is expected to be the fastest growing in the respiratory syncytial virus vaccines market, as this vaccine is witnessing fast expansion, which is being driven by the disease load, improving healthcare infrastructure, and the aging population. Industry foretells and expects a main CAGR, which is fueled by developing awareness and current regulatory checks in main countries like Japan, China, and Singapore.
A bigger understanding of this toxic health risk of the RSV has recommended exceptional populations, which include older adults and infants, too, leading to the urge for preventive solutions. Governments in the Asia Pacific countries are excessively focused on developing healthcare and expanding immunization programs, particularly for children.
India Respiratory Syncytial Virus Vaccines Market Trends:
The trend for the respiratory syncytial virus (RSV) immunization in India is transforming from not at all locally checked vaccines to the current revelation of monoclonal antibody (mAb) therapies for infants. While there are no active RSV vaccines that are now available in India for any age group, the industry for passive immunization is developing rapidly, with the main area of growth being in this sector.
The Central Drugs Standard Control Organization (CDSCO) has officially granted the marketing approval for Sanofi’s Beyfortus in India in June 2024. This serves as the latest protective diagnosis for the newborns and infants in their first RSV season and for the high-risk toddlers up to 24 months.
Need a Tailored Version of the Report? | Get Customization Options Here: https://www.visionresearchreports.com/report/customization/41757
Respiratory Syncytial Virus Vaccines Market Segmental Analysis
Type Analysis
How did the Passive Immunization Segment Dominate the Respiratory Syncytial Virus Vaccines Market?
The passive immunization has dominated the segment in 2024, as at the time of newborn status in the delivery room, after admission of vitamin K, parents are again reminded that passive immunization against the RSV is still suggested during hospitalization in the luxury time. The medical team, nursing staff, and the midwives on the maternity ward will constantly clear any queries parents may have.
For instance, to this,
• In June 2025, Merck, which is known as MSD outside the United States and Canada, officially revealed that the U.S. Food and Drug aMINISTRATION (FDA) has checked ENFLONSIA for the protection of respiratory syncytial virus (RSV), which is lower respiratory tract disease in neonates (newborns) and the infants who are born at the time of the first RSV season.
The Preventive Vaccine Segment is Predicted to Rise at the Fastest Rate in the Respiratory Syncytial Virus Vaccines Market.
Nirsevimaba and clesrovimab are kinds of monoclonal antibody products that are passive immunisation. These are not so officially vaccines, but in a regular sense (active immunization), they are being utilised in a way similar to routine childhood vaccines and may be recommended as vaccines by some entries. Nirsevimab and clesrovimab fight against long-standing prevention from the RSV that indirectly protects and is predicted to last for at least 5 months (which is about the length of a regular RSV season). The Nirsevimab and the clesrovimab are part of the vaccines for the children program.
Technology Analysis
Why did the Monoclonal Antibodies (mAbs) Segment Dominated the Respiratory Syncytial Virus Vaccines Market?
The monoclonal antibodies segment has dominated the market in 2024 as respiratory syncytial virus has passive immunization programs that focus on the protection during the first 6 months of life, which can linearly lower the RSV disease burden. Palivizumab is currently the most commonly used prophylaxis to prevent RSV disease in infants. Two meta-analyses of the randomized clinical trials have shown that palivizumab could mainly reduce the RSV-linked hospitalizations by 51 to 55 % 1000 candidates.
Also, the latest monoclonal antibodies (mAbs) have been created, such as nirsevimab, that definitely protect the infant from the RSV-linked hospitalization and infection during a complete RSV season with a single dose.
The Recombinant protein Integrated with Adjuvant Segment is predicted to rise at the Fastest Rate in the Respiratory Syncytial Virus Vaccines Market.
The recombinant proteins play an important role in making new vaccines, diagnostic tools, and therapies for the respiratory syncytial virus. The pumps in use concentrate on the RSV fusion and the attachment (G) glycoproteins, which are the initial targets for the neutralising antibodies.
Research using the recombinant G protein has shown that it can develop the particular T-cell feedback. For instance, a recombinant G protein has been integrated with a baculovirus-expressed M2 protein that has been shown to elicit a rigid immune response in animal models.
Targeted Population Analysis
Why did the Infants and Children Segment dominate the Respiratory Syncytial Virus Vaccines Market?
The infants and the children have dominated the market in 2024 as respiratory syncytial virus (RSV) is a seasonal virus that spreads conveniently among children and babies. For several kids, RSV feels like a cold. Hence, RSV can sometimes point to complexities that cause many symptoms, such as trouble breathing. RSV in infants (the babies who are younger than 1 year) is particularly hard. It is the most prevalent reason for hospitalisation in infants. And, for several kids, the RSV is silent and goes away with at-home care, like other colds. But younger kids may have more serious symptoms.
It conveniently propagates among kids in particular group settings like daycares and schools.
With instance to this,
• Respiratory syncytial virus (RSV) is one of the most prevalent reasons for lower respiratory infections in children worldwide, as well as leading to the overall load of many respiratory diseases among elderly persons.
• Every year, RSV leads to 3.6 million hospitalizations and about 100,000 deaths in children under the age of 5 years old in low and middle-income countries that have restricted access to assistive medical care.
The Adult Segment is Predicted to Rise at the Fastest Rate in the Respiratory Syncytial Virus Vaccines Market.
RSV is a prevalent virus that we may have definitely been exposed to before. One might not have known that we can undergo RSV without a lab test in order to confirm the treatment, as it is generally lumped collectively with other respiratory infections, and it causes cold-like symptoms.
Respiratory Syncytial virus has the complete capability to make anyone fall ill. Generally, as adults, when we become sick with RSV, we get mild cold symptoms like a sore throat, runny nose, cough, and headache.
Distribution Channel Analysis
Why did the Hospital and Retail Pharmacies Segment dominate the Respiratory Syncytial Virus Vaccines Market?
The hospital and retail pharmacies have dominated the market in 2024 as they are both main distribution channels for the virus vaccines, as they have the building, public trust, and staff, which is essential for smooth and safe mass immunization. Just like temporary vaccination sites, hospitals serve a relevant and managed surrounding, which is important for carrying fragile biological products and tracking the complicated vaccination campaigns.
Also, vaccines should be stored within a particular temperature range, which is known as “Cold Chain, “to stay effective. Hospitals are relevant, tailored refrigeration and the freeze machine, which can meet strict temperature needs and demands.
The Government Suppliers Segment is Predicted to Rise at the Fastest Rate in the Respiratory Syncytial Virus Vaccines Market.
The government suppliers are a main distribution channel for the virus vaccines due to their role in managing, purchasing, and serving large quantities of vaccines as a public good. This method ensures huge access, particularly for the necessary and routine immunizations, which are frequently available at no cost to the public.
Governments, as shown by companies like the World Health Organization (WHO), give importance to providing vaccines to everyone regardless of socio-economic status. This is important for managing infectious diseases as widespread vaccination can lead to community (herd) immunity.
Browse More Insights:
• Vaccine Market: https://www.visionresearchreports.com/vaccine-market/41731
• Personalized Cancer Vaccine Market: https://www.visionresearchreports.com/personalized-cancer-vaccine-market/41670
• Pneumococcal Vaccine Market: https://www.visionresearchreports.com/pneumococcal-vaccine-market/41294
• Dog Vaccine Market: https://www.visionresearchreports.com/dog-vaccine-market/40961
• Virus Filtration Market: https://www.visionresearchreports.com/Virus-filtration-market/41685
• Human Immunodeficiency Virus Therapeutics Market https://www.visionresearchreports.com/human-immunodeficiency-virus-therapeutics-market/40869
Recent Developments in the Respiratory Syncytial Virus Vaccines Market
• In February 2025, AIM Vaccine Co.Ltd, a top PRC vaccine organization, had revealed that its independently created mRNA RSV(respiratory syncytial virus) vaccine had been approved for clinical trials by the U.S. Food and Drug Administration (FDA).
• In June 2025, GSK plc had recently disclosed that the European Medicines Agency had approved the organization’s regulatory usage to extend the usage of its adjuvanted recombinant respiratory syncytial virus vaccine to include adults aged 18 years of age.
• In June 2025, GSK plc officially revealed that Japan’s Ministry of Health, Labour and Welfare( MHLW) had used the organization's regulatory application to expand the use of its adjuvanted recombinant respiratory syncytial virus vaccine.
• In April 2025, Pfizer declared that the U.S. centres for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) had officially voted to expand its recommendations for the use of respiratory syncytial virus vaccines for adults aged between 5059 years at growing risk of RSV-linked lower respiratory tract disease.
• In September 2025, Nova Scotia has funded USD6.9 Million to introduce a new program in order to vaccinate or protect infants and some seniors against the respiratory syncytial virus.
Respiratory Syncytial Virus Vaccines Market Key Companies
• Pfizer Inc
• GSK plc
• AstraZeneca plc
• Moderna ,Inc
• Sanofu S.A
• Novavax, Inc
• Bavarian Nordic A/S
• Johnson &Johnson (Janssen Pharmaceuticals)
• Merck & Co, Inc
• Melissa Vaccines, Inc
Respiratory Syncytial Virus Vaccines Market Segmentations:
By Type
• Passive Immunization
• Preventive Vaccines
By Technology
• Monoclonal Antibodies
• Recombinant Protein + Adjuvant
• mRNA-Based Vaccine
• Virus-Like Particle (VLP)
By Targeted Population
• Adults
• Infants and children
By Distribution Channel
• Hospital & Retail Pharmacies
• Government Suppliers
• Others
By Regional
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East and Africa (MEA)
Instant Delivery Available | Purchase This Exclusive Research Report Now: https://www.visionresearchreports.com/report/checkout/41757
You can place an order or ask any questions, please feel free to contact at: sales@visionresearchreports.com
About Us
Vision Research Reports is a premier service provider offering strategic market insights and solutions that go beyond traditional surveys. We specialize in actionable market research, delivering in-depth qualitative insights and strategies to global industry leaders and executives, helping them navigate future uncertainties. Our offerings include consulting services, syndicated market studies, and bespoke research reports.
We are committed to excellence in qualitative market research, fostering a team of experts with deep industry knowledge. Our goal is to help clients understand both current and future market trends, empowering them to expand their portfolios and achieve their business objectives with the right guidance.
Web: https://www.visionresearchreports.com
Our Trusted Data Partners
Precedence Research | Statifacts | Nova One Advisor
For Latest Update Follow Us: LinkedIn

