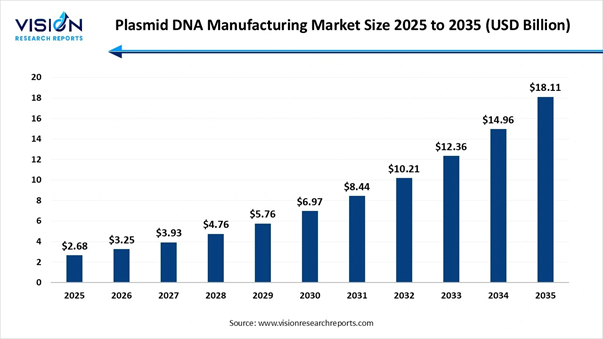
The global plasmid DNA manufacturing market size is valued at USD 2.68 billion in 2025 and is predicted to increase from USD 3.25 billion in 2026 to reach around USD 18.11 billion by 2034, registering a robust CAGR of 21.05% from 2025 to 2034, Study Published by Vision Research Reports.
The increasing demand for plasmid DNA is driven by its essential role in advanced therapies, including gene therapy, cell therapy, and DNA vaccines such as CAR-T therapeutics. In addition, the surge in biotechnology investments, rising clinical trials, and increasing prevalence of genetic disorders and cancers are expected to further drive the demand for plasmid DNA.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Preview the Report Before You Buy – Get Sample Pages 👉 https://www.visionresearchreports.com/report/sample/39735
What is Plasmid DNA Manufacturing?
Plasmid DNA manufacturing is a critical process in biotechnology and research that enables the production of DNA constructs. These constructs are widely used in genetic manipulation for applications in medicine, molecular biology, and agriculture. Key purposes of pDNA include:
• Gene editing
• Gene expression studies
• Protein production
The demand for plasmid DNA varies by application and quality requirements. For instance, GMP-grade plasmids are essential for clinical trials and commercial therapeutics, while research-grade plasmids are used for preclinical studies, laboratory research, and academic purposes. Companies may also use contract development and manufacturing organizations (CDMOs) to source either research-grade or GMP-grade plasmids.
Plasmid DNA Manufacturing Market Key Highlights:
• By region, North America led the market with the largest share of 42.5% in 2025.
• By region, Asia Pacific is expected to expand at the highest CAGR from 2026 to 2035.
• By development phase, the clinical therapeutics contributed the largest market share of 55% in 2025.
• By application, the cell & gene therapy captured the maximum market share of 55% in 2025.
• By disease, the cancer generated the maximum market share of 41% in 2025.
• By grade, the GMP grade registered the maximum market share of 87% in 2025.
Leading DNA Vaccine Companies and Their Offerings
|
Company |
Key Offerings |
|
Inovio Pharmaceuticals (USA) |
Leading company in human DNA vaccine development. |
|
Cadila Healthcare (Zydus Cadila – India, Japan) |
Developer of ZyCoV-D, the first DNA vaccine approved for human use against SARS-CoV-2. |
|
Osaka University / AnGes (Japan) |
Collaborative efforts have led to promising DNA vaccine candidates. |
|
Genexine Consortium (South Korea) |
Leading DNA vaccine development initiatives in Asia. |
|
Takis & Rottapharm Biotech (Italy) |
Developed the Covid-eVax DNA vaccine candidate. |
Latest Trends in the Plasmid DNA Manufacturing Market
• Continuous Manufacturing: Continuous production processes are being adopted to increase yields, reduce costs, and maintain consistent quality compared to batch manufacturing.
• Single-Use Technologies: Disposable bioreactors and fluid management systems reduce cross-contamination risks and lower cleaning costs, enhancing operational flexibility.
• Automation and Artificial Intelligence: AI-assisted automation optimizes pH, temperature, and nutrient flow in real-time, increasing yields while ensuring GMP compliance.
• Advanced Purification Techniques: Innovations in filtration and chromatography enhance the removal of impurities such as genomics DNA, proteins, and endotoxins, improving product quality.
• Clinical and Commercial-Scale Production: Demand for GMP-grade plasmid DNA is increasing for clinical trials and commercial production of cell and gene therapies.
• Focus on Oncology and Gene Therapy: Plasmid DNA is critical for viral vector production, which is widely used in cancer immunotherapies and gene therapy.
Discover the Full Market Insights 👉
https://www.visionresearchreports.com/plasmid-dna-manufacturing-market/39735
Plasmid DNA Manufacturing Market Dynamics
Opportunities
Plasmid DNA offers significant opportunities in advanced therapies. Emerging technologies, such as cell-free DNA manufacturing, provide a controlled, efficient, and scalable method for producing plasmids. These platforms are particularly beneficial for mRNA-based therapeutics and vaccines, enabling faster development cycles. As synthetic plasmid production expands, regulatory standards tailored to these technologies are expected to evolve.
Key Challenges
Despite advances, plasmid manufacturing faces challenges:
• Stage-based production may limit flexibility when switching between different plasmids or scaling up production.
• High costs and regulatory requirements remain significant barriers, particularly for GMP-grade plasmids used in viral and RNA vector production.
Technological Advancements
• Plasmid DNA plays a central role in cell and gene therapies, mRNA synthesis, viral vectors, and DNA vaccines.
• GMP-grade plasmids are required for both veterinary and human medicine applications, ensuring compliance with stringent regulatory standards.
• Advanced production facilities with strong quality management systems are essential for reliable plasmid manufacturing.
Government Support and Initiatives
•
Bionova Scientific Investment (USA): In June 2024, Bionova invested USD
100 million to expand plasmid DNA manufacturing, including a new 100,000
sq. ft. facility in The Woodlands, Texas.
• BioE3 Policy (India): A government initiative to transform India into
a global biotech hub by supporting high-performance biomanufacturing.
•
National Biopharma Mission (India): Promoted by the Department of Biotechnology and BIRAC,
this initiative supports vaccine, biopharmaceutical, and medical device
development.
• European Union & Horizon Europe Programs: Supporting innovative
biomanufacturing technologies, including plasmid DNA production for gene therapy and vaccine research.
Plasmid DNA Manufacturing Market Report Coverage
|
Report Attribute |
Key Statistics |
|
Market Size in 2026 |
USD 3.25 Billion |
|
Market Size in 2027 |
USD 3.93 Billion |
|
Market Size in 2031 |
USD 8.44 Billion |
|
Market Size in 2033 |
USD 12.36 Billion |
|
Market Size by 2035 |
USD 18.11 Billion |
|
Growth rate from 2025 to 2034 |
CAGR of 21.05% |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2034 |
|
Segments Covered |
By Disease, By Grade, By Application, By Development Phase |
|
Companies Covered |
Charles River Laboratories, VGXI, Inc., Danaher (Aldevron), Kaneka Corp., Nature Technology, Cell and Gene Therapy Catapult, Eurofins Genomics, Lonza, Luminous BioSciences, LLC, and Akron Biotech. |
For Orders or Inquiries, Don’t Hesitate to Reach Out: sales@visionresearchreports.com
Plasmid DNA Manufacturing Market Regional Analysis
North America to Sustain its Dominance
North America dominates the global market due to the presence of well-established biotechnology infrastructure, leading pharmaceutical companies, and extensive R&D investment. The U.S. drives market growth with its advanced clinical trial ecosystem, regulatory frameworks for gene and cell therapies, and increasing adoption of GMP-grade plasmid DNA. Canada is also contributing through biotech startups and research institutions focused on DNA vaccines and gene therapy.
The U.S. is well-positioned to remain the global leader for pDNA manufacturing through the next decade. Continued investment in capacity expansion, adoption of single-use and automated manufacturing technologies, and strong regulatory compliance will be key to meeting growing global demand from gene‑ and cell‑therapy developers worldwide.
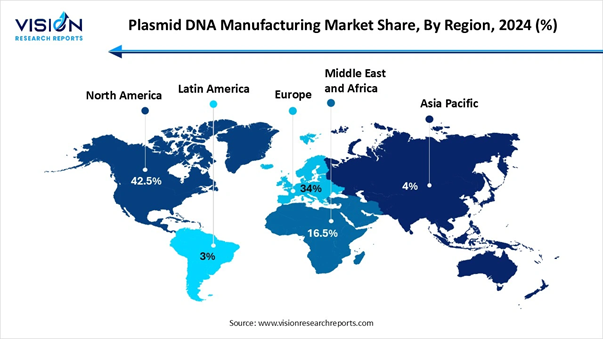
Asia Pacific Plasmid DNA Manufacturing Market Analysis
Asia-Pacific is the fastest-growing region, led by India, China, Japan, and South Korea. Rapid expansion of biotechnology research, government initiatives to promote biomanufacturing, and increasing DNA vaccine production contribute to strong market growth. India’s BioE3 policy and National Biopharma Mission exemplify government support, while China invests heavily in gene therapy R&D and GMP-grade plasmid production.
India to Boom Rapidly Till 2030
India is rapidly emerging as a key hub for plasmid DNA manufacturing and broader biomanufacturing, thanks to strong government initiatives, growing biotech investment, and rising demand for gene‑ and cell‑therapy development.
• Government support & policy environment: India’s biotech ambitions are being accelerated by policies such as BioE3 Policy (Biotechnology for Economy, Environment and Employment) and National Biopharma Mission. These frameworks aim to build India into a global‑scale biomanufacturing center, supporting vaccines, gene therapy, diagnostics and advanced biologics. This creates favourable regulatory and funding climate for pDNA manufacturing.
• Growing domestic demand: With rising interest in DNA vaccines, gene therapies and cell therapies (for cancer, genetic disorders, infectious diseases), local demand for GMP‑grade plasmid DNA is growing. Indian biotech firms and CDMOs are increasingly focusing on building pDNA capacity to support both clinical trials and eventual commercialization.
Europe holds a significant share, supported by countries such as Germany, the UK, France, and Italy. Government funding, strong healthcare infrastructure, and advanced biopharmaceutical manufacturing capabilities drive demand. The EU’s focus on regulatory harmonization and programs like Horizon Europe provides incentives for innovation in plasmid DNA and gene therapy technologies.
Need a Tailored Version of the Report? | Get Customization Options Here: https://www.visionresearchreports.com/report/customization/39735
Plasmid DNA Manufacturing Market Segmentation Analysis
Development Phase Analysis
The clinical therapeutics segment dominated the plasmid DNA manufacturing market in 2025, as plasmid DNA (pDNA) production has gained importance for clinical therapeutics because of its foundational role in the growth of gene therapy, mRNA vaccines, and DNA vaccines. While the plasmids were beginning a machine for recombinant protein manufacturing, that are developed growth, leading to increased understanding of the immune feedback and production improvements that have updated them into important starting materials for a huge series of advanced medicines.
The pre-clinical therapeutics segment is predicted to rise at the fastest rate. The pre-clinical plasmid DNA manufacturing serves heavy purity, less endotoxin plasmid DNA, particularly crafted for the pre-clinical growth of advanced therapeutic modalities, such as gene therapies and DNA vaccines. Furthermore, pre-clinical plasmid DNA is perfect for preclinical toxicology and safety research by using the large animal models. As this service uses the same stage production technology as VGXI’s cGMP manufacturing service, the pre-clinical plasmid manufacturing ensures scalability to align with clinical demand as projects mature.
Application Analysis
The cell and gene therapy segment has dominated the plasmid DNA manufacturing industry in 2025, as good-quality plasmid DNA is a main element in cell and gene therapy production and, as such, is in high demand. This has led to the demand for updated production to align with the urge for volume, as well as the quality needed for the supply in the production of therapeutics.
In some scenarios, plasmids can be utilised directly as non-viral vectors for gene delivery. They are being crafted to carry a therapeutic gene which can be delivered into a patient’s cells via procedures like electroporation and injection.
The DNA vaccines segment is expected to rise at the fastest rate in the plasmid DNA manufacturing market. DNA vaccines are an area of nucleic acid-dependent immunization method that totally depends on a plasmid DNA molecule crafted to encode one or more antigenic proteins. Once they are entered, the plasmid penetrates hist cells as many of the noticed antigen-serving cells (APCs such as dendritic cells and upon handling the nucleus, it is being changed into messenger RNA (mRNA) which is then translated into the antigen protein.
Disease Analysis
The cancer segment dominated the market in 2025 as cancer remains the biggest space making for plasmid DNA and given the pace of growth in terms of oncology, this domination will develop over the long term. DNA plasmids have been explored for usage in cancer treatment medicines, as this disease stands at the core of vaccines that have already showcased their capability to generate the immune system to fight cancer cells, and the usage of DNA plasmids in cancer gene therapy can quickly direct protein formulation in order to avoid those cancer cells.
The genetic disorder segment is expected to rise at the fastest rate in the plasmid DNA manufacturing market. The crucial application of the plasmid DNA is in the manufacturing of man-made proteins. These proteins are utilised in vaccines, pharmaceuticals, and medical research. By filling the particular genetic series into the plasmid DNA, researchers can count bacterial or mammalian cells to generate large proteins. This procedure plays an important role in mRNA manufacturing, that are important for therapeutic and vaccine growth.
Also, the plasmid DNA plays an important role in biotechnology as it serves as a rigid machine for medical advancements and genetic engineering.
Grade Analysis
The GMP segment has dominated the plasmid DNA manufacturing market in 2025, as plasmid design is the primary element in successful GMP plasmid production. It directly affects the scalability, yields, and the overall product quality. A qualified CDMO should definitely provide expert assistance in the plasmid design, which helps users in developing constructs that are overall reliable with the GMP range-up and present perfect production practices, named the cGMP biologics manufacturing needs and demands from the beginning of the design.
The R&D grade segment is expected to rise at the fastest rate in the plasmid DNA manufacturing market. Synthetic DNA is widely used and is available as the research grade, as some suppliers, such as Touchlight and 4basebio, serve further intermediate grades that are perfect for good laboratory practice or the investigational new drug (IND) use, which allows studies. Also, research-grade DNA is manufactured under standard laboratory surroundings without rigid quality checking of the production procedure or the above comprehensive documentation, and is proposed for usage in non-human uses and research.
Browse More Insights:
• Viral Vector and Plasmid DNA Manufacturing Market: https://www.visionresearchreports.com/viral-vector-and-plasmid-dna-manufacturing-market/41584
• Targeted DNA RNA Sequencing Market: https://www.visionresearchreports.com/targeted-dna-rna-sequencing-market/41577
• DNA and Gene Cloning Services Market: https://www.visionresearchreports.com/dna-and-gene-cloning-services-market/41518
• U.S. Plasmid DNA Manufacturing Market: https://www.visionresearchreports.com/us-plasmid-dna-manufacturing-market/41367
• DNA Diagnostics Market: https://www.visionresearchreports.com/dna-diagnostics-market/40777
Recent Developments in the Plasmid DNA Manufacturing Market
• In April 2025, Probio, which is a worldwide contract development and manufacturing organization 9CDMo), is delighted to reveal the launch of its GMP Plasmid DNA Manufacturing service at its state-of-the-art facility in Hopewell.
• In November 2025, Bharat Biotech International Ltd, which is a leading player in the Indian pharmaceutical sector, created a major shift into the rapidly developing field of cell and gene therapy. The organization has revealed Nucelion Therapeutics Pvt Ltd, which is completely owned by a Contract Research, Development and Manufacturing Organisation, which is concentrated on cell and gene therapy growth and manufacturing.
• In August 2025, Bionova Scientific, which is a full-service biologics contract development and production organization (CDMO) and also an option of global conglomerate Asahi Kasei, disclosed the opening of the 10,000 sqft cutting-edge plasmid DNA (pDNA ) growth and manufacturing facility in the Woodlands, Texas.
• In September 2025, Elegen, which is a top company in cell-free DNA production, officially revealed ENFINIA IVT Ready DNA. This inventive product is crafted for quick usage in vitro transcription 9IVT) that lowers the time needed for developing the mRNA-based therapeutics by weeks.
• In January 2025, Aldevron, a top company in the manufacturing of DNA, protein, and RNA, revealed its current invention, named Alchemy, which is a cell-free DNA technology. This updated technology shows significant advancements in synthetic DNA production, which serves as a cell-free and enzymatic procedure to make linear DNA designs for in vitro transcription synthesis of the mRNA molecules.
• In July 2025, Purilogics, which is a Donaldson Life Sciences organization, is an expert in the biopharmaceutical membrane chromatography growth, which disclosed the availability of its initial production-grade product within its Purexa NAEX technology.
Plasmid DNA Manufacturing Market Top Companies
• Charles River Laboratories: A global leader in preclinical and clinical research services, Charles River provides high-quality plasmid DNA manufacturing through its integrated biologics solutions. The company supports advanced therapy developers with end-to-end GMP and non-GMP plasmid production.
• VGXI, Inc.: VGXI specializes in high-purity plasmid DNA manufacturing for DNA vaccines, gene therapies, and immunotherapies. Known for its proprietary plasmid production technologies, the company delivers both GMP and research-grade plasmids with industry-leading yields.
• Danaher (Aldevron): Aldevron, part of Danaher, is one of the largest global producers of plasmid DNA, viral vectors, and mRNA. It is widely recognized for its GMP-grade plasmids that support gene therapy, vaccine, and CRISPR applications.
• Kaneka Corp.: Kaneka offers CDMO services for plasmid DNA production, focusing on high-quality, scalable biomanufacturing solutions. Its molecular biotechnology division supports both clinical and commercial-scale genetic medicine programs.
• Nature Technology (NTC): Nature Technology provides advanced plasmid design and manufacturing tools, including its proprietary HyperGRO™ fermentation process. The company is known for technology-driven solutions that enhance plasmid yield and quality.
• Cell and Gene Therapy Catapult: Based in the UK, this organization accelerates the development and commercialization of cell and gene therapies by offering advanced manufacturing, analytical testing, and regulatory support, including plasmid and vector-related expertise.
• Eurofins Genomics: Eurofins Genomics supplies custom plasmid DNA production services for research, diagnostics, and therapeutic applications. Its global infrastructure allows rapid turnaround and high-quality plasmid preparation.
• Lonza: Lonza is a leading CDMO offering large-scale GMP plasmid DNA manufacturing for gene therapy, mRNA vaccines, and cell therapy developers. The company is known for its robust regulatory compliance and commercial-scale capabilities.
• Luminous BioSciences, LLC: Luminous BioSciences focuses on customized plasmid DNA solutions for early-stage R&D, preclinical studies, and therapeutic development. The company provides flexible, high-quality plasmid design and production services.
• Akron Biotech: Akron Biotech manufactures critical raw materials for advanced therapies, including plasmid DNA used in viral vector and mRNA production. Its GMP-compliant platforms support both clinical and commercial cell and gene therapy programs.
Plasmid DNA Manufacturing Market Segmentations:
By Grade
• R&D Grade
• GMP Grade
By Development Phase
• Pre-Clinical Therapeutics
• Clinical Therapeutics
• Marketed Therapeutics
By Application
• DNA Vaccines
• Cell & Gene Therapy
• Immunotherapy
• Others
By Disease
• Infectious Disease
• Cancer
• Genetic Disorder
• Others
By Region
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East & Africa
Instant Delivery Available | Purchase This Exclusive Research Report Now: https://www.visionresearchreports.com/report/checkout/39735
You can place an order or ask any questions, please feel free to contact at: sales@visionresearchreports.com
About Us
Vision Research Reports is a premier service provider offering strategic market insights and solutions that go beyond traditional surveys. We specialize in actionable market research, delivering in-depth qualitative insights and strategies to global industry leaders and executives, helping them navigate future uncertainties. Our offerings include consulting services, syndicated market studies, and bespoke research reports.
We are committed to excellence in qualitative market research, fostering a team of experts with deep industry knowledge. Our goal is to help clients understand both current and future market trends, empowering them to expand their portfolios and achieve their business objectives with the right guidance.
Web: https://www.visionresearchreports.com
Our Trusted Data Partners
Precedence Research | Statifacts | Nova One Advisor
For Latest Update Follow Us: LinkedIn
Discover More Market Trends and Insights from Vision Research Reports:
• Biologics Manufacturing Market: https://www.visionresearchreports.com/biologics-manufacturing-market/41782
• Non-viral Gene Delivery Technologies Market: https://www.visionresearchreports.com/non-viral-gene-delivery-technologies-market/41780
• Trauma Care Centers Market: https://www.visionresearchreports.com/trauma-care-centers-market/41778
• U.S. mHealth Apps Market: https://www.visionresearchreports.com/us-mhealth-apps-market/41776
• Surgical Instrument Tracking Systems Market: https://www.visionresearchreports.com/surgical-instrument-tracking-systems-market/41777
• Hospital Electronic Health Records Market: https://www.visionresearchreports.com/hospital-electronic-health-records-market/41775
• U.S. Large and Small-scale Bioprocessing Market: https://www.visionresearchreports.com/us-large-and-small-scale-bioprocessing-market/41774
• U.S. Antibody Drug Conjugates Market: https://www.visionresearchreports.com/us-antibody-drug-conjugates-market/41773
• U.S. Medical Tourism Market: https://www.visionresearchreports.com/us-medical-tourism-market/41772

