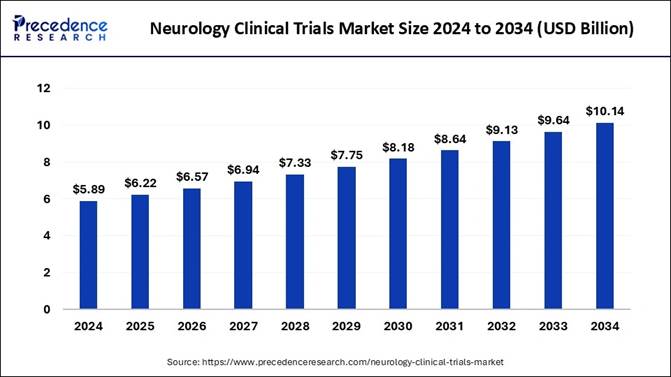
The global neurology clinical trials market size is expected to reach nearly USD 10.14 billion by 2034 increasing from USD 6.22 billion in 2025 and is growing at a CAGR of 5.58% from 2025 to 2034.
According to Precedence Research, the neurology clinical trials market was estimated at USD 5.89 billion in 2024. It is predicted to grow from USD 6.22 billion in 2025 to USD 10.14 billion by 2034. The benefits of neurology clinical trials include access to the latest therapies, reduced cost, a chance to help others, closer monitoring, and careful and regular monitoring from the research team that includes healthcare professionals, doctors, and others. These factors help to the growth of the market.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/4657
Neurology Clinical Trials Market Highlights:
• In terms of revenue, the neurology clinical trials market accounted for USD 6,570 million in 2024.
• North America contributed the highest market share of 48% in 2024.
• Asia Pacific is projected to grow at a strong CAGR of 5.93% from 2025 to 2034.
• By phase, the phase II segment has generated more than 47% of market share in 2024.
• By phase, the phase III segment is projected to expand at a CAGR of 5.62% from 2025 to 2034.
• By indication, the epilepsy segment captured the highest market share of 23% in 2024.
• By indication, Huntington’s disease segment is growing at a CAGR of 6.04% from 2025 to 2034.
• By study design, the interventional generated the biggest market share of 96% in 2024.
• By study design, the observational segment is expected to grow at a CAGR of 5.83% from 2025 to 2034.
Neurology Clinical Trials Market Overview and Industry Potential
From Awareness to Action: The Surge in Neurology Clinical Trials
The neurology clinical trials market is expected to see rapid growth owing to the number of patients who are suffering from brain and nervous system disorders. Furthermore, by having limited treatment options, the need for innovative medicine has increased in recent years. Furthermore, factors such as the development of low-cost treatments and awareness of these diseases are likely to provide a huge industry share in the coming years.
Also Read@ Neurology Market Advancing Precision Care for Neurological Disorders Worldwide
Major Tech-advancements in Neurology Clinical Trials Market:
1) AI-Powered Patient Recruitment Platforms: Artificial intelligence is being used to match eligible patients to neurology trials more accurately and rapidly, improving enrollment speed and diversity.
2) Remote Monitoring & Decentralized Trials: Wearable devices and mobile health platforms enable real-time neurological data capture, allowing trials to be conducted remotely and increasing patient retention.
3) Biomarker-Driven Trial Design: Advances in genomic and proteomic biomarkers are helping identify patient subgroups and predict treatment responses in disorders like Alzheimer’s and Parkinson’s.
4) Digital Cognitive Assessment Tools: Digital neuropsychological tools and gamified apps are being used to assess cognitive function more objectively and frequently in clinical settings.
Browse Detailed Insight@ https://www.precedenceresearch.com/neurology-clinical-trials-market
Case Study: Decentralized Alzheimer’s Clinical Trial Using AI-Powered Monitoring
In 2023, a leading global Contract Research Organization (CRO) in partnership with top neurology hospitals launched a Phase II decentralized clinical trial for Alzheimer’s disease. Traditionally, Alzheimer’s trials face significant challenges: low patient enrollment rates, high dropout levels, and difficulties in continuous cognitive monitoring. To address these issues, the study deployed a combination of AI-driven patient recruitment tools, wearable devices, and mobile health applications.
🔹AI Recruitment: Artificial intelligence platforms were used to scan electronic health records and match eligible patients to trial criteria. This reduced the enrollment cycle by nearly 40% compared to conventional recruitment.
🔹Remote Monitoring: Patients were provided with wearable devices capable of tracking sleep patterns, movement, and neurological activity. Data was transmitted in real-time to trial investigators, ensuring early detection of anomalies and reducing the need for frequent in-person visits.
🔹Digital Cognitive Assessments: Instead of relying solely on scheduled clinical visits, patients completed gamified neuropsychological assessments at home, generating more frequent and objective cognitive function data.
Outcomes:
🔹Patient enrollment increased by 35% with a more diverse demographic mix.
🔹Retention rates improved by 25% due to reduced travel burden and patient-friendly trial design.
🔹Real-time data allowed faster decision-making for trial investigators, cutting down delays in evaluating treatment safety and efficacy.
This case demonstrates how technology-driven clinical trials can overcome long-standing barriers in neurology research, particularly in complex diseases like Alzheimer’s. It highlights the industry shift toward patient-centric, decentralized, and data-driven approaches, aligning directly with the broader growth of the neurology clinical trials market.
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
What is Major Potential in Neurology Clinical Trials Market?
High Value Growth Awaits in Rare Neurological Disease Therapeutics
The development of treatment for rare neurological disorders is anticipated to create lucrative opportunities for manufacturers in the coming years. Furthermore, the manufacturer can focus on diseases like Huntington’s disease and others, where the treatments are very rare and expensive. By establishing collaborations with pharmaceutical companies and research centres, the manufacturers are likely to gain long-term profit margins with a sophisticated consumer base in the coming years.
What Holds the Neurology Clinical Trials Market Behind?
The Financial Strain Behind Neurological Clinical Trial Development
The higher production cost of the neurological clinical trials is anticipated to hinder the industry's growth during the forecast period. Moreover, the development of these therapies requires complex biotechnology procedures and stricter regulatory approvals, which are expected to increase the expense in the development of these antibodies. This higher cost can create growth hurdles for the new market entrants and mid-sized businesses, where the limited budget is the primary factor.
Global Clinical Trials Market Size and Growth Outlook (2025 to 2034)
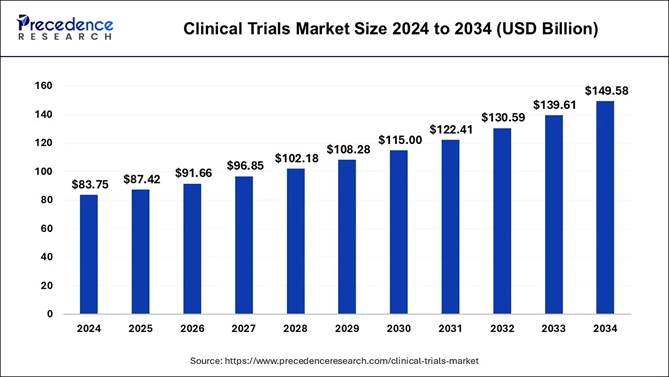
The neurology clinical trials market is a vital subsegment of the global clinical trials market, which was valued at USD 83.75 billion in 2024 and is projected to reach USD 149.58 billion by 2034, expanding at a CAGR of 6.10%.
The broader clinical trials market spans multiple therapeutic areas—including oncology, cardiovascular, infectious diseases, and neurology—and its expansion is being fueled by rising R&D investments, regulatory support for accelerated approvals, and the adoption of decentralized and technology-enabled trial designs. Within this landscape, neurology stands out as one of the fastest-growing segments, driven by the urgent demand for innovative treatments for Alzheimer’s, Parkinson’s, epilepsy, and other neurodegenerative disorders.
Clinical Trials Market Key Takeaways
• In terms of revenue, the global clinical trials market accounted for USD 87.42 billion in 2024.
• It is projected to reach USD 149.58 billion by 2034.
• The market is expected to grow at a CAGR of 6.10% from 2025 to 2034.
• North America has held 59.31% of the total market share in 2024.
• Asia Pacific region is growing at a CAGR of 7.16% during the forecast period.
• By study design, the interventional study segment has captured 70.50% market share in 2024.
• By study design, the expanded access segment is expected to grow at a solid CAGR of 8.20% during the forecast period.
• By indication, the oncology segment captured the biggest market share of 31.70% in 2024.
• By indication, the CNS conditions segment is projected to grow at a solid CAGR of 7.60% during the forecast period.
• By service type, the laboratory services segment generated the major market share of 24.60% in 2024.
• By service type, the decentralized clinical trial services segment is expected to expand at a solid CAGR of 9.40% during the forecast period.
The Complete Study is Immediately Accessible | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/1185
Scope of Neurology Clinical Trials Market
|
Report Coverage |
Details |
|
Market Size in 2024 |
USD 5.89 Billion |
|
Market Size in 2025 |
USD 6.22 Billion |
|
Market Size in 2031 |
USD 8.64 Billion |
|
Market Size by 2034 |
USD 10.14 Billion |
|
CAGR 2025 to 2034 |
5.58% |
|
Leading Region in 2024 |
North America |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2034 |
|
Segments Covered |
Phase, Study Design, Indication, Study Design Indication, Phase Indication, and Regions |
|
Regions Covered |
North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
➡️ Become a valued research partner with us ☎ https://www.precedenceresearch.com/schedule-meeting
Neurology Clinical Trials Market Key Regional Analysis:
What Makes North America the Leader in Neurological Clinical Trials ?
North America held the dominant share of the neurology clinical trials market in 2024, owing to advanced healthcare infrastructure and modern technology innovation. Moreover, factors such as high funding availability and enlarged patient involvement are contributing to the industry's growth in recent years.
Furthermore, the higher prevalence of neurological diseases is significantly driving the industry as individuals are actively demanding innovative treatments for Alzheimer's and Parkinson’s diseases.
How Big is the U.S. Neurology Clinical Trials Market?
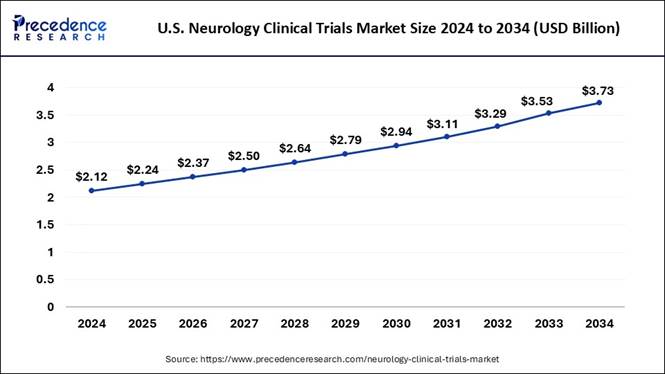
The U.S. neurology clinical trials market size will grow from USD 2.24 billion in 2025 to approximately USD 3.73 billion by 2034, expanding at a CAGR of 5.81% from 2025 to 2034.
Note: This report is readily
available for immediate delivery. We can review it with you in a meeting to
ensure data reliability and quality for decision-making.
📥 Download Sample Pages
for Informed Decision-Making 👉 https://www.precedenceresearch.com/sample/4657
Key Drivers of U.S. Neurology Clinical Trials Market:
1) Market Leadership & Maturity: The U.S. is the global leader in neurology clinical trials due to its advanced healthcare infrastructure, strong regulatory framework, and high research funding.
2) High Volume of Trials & Approvals: A large share of global neurology clinical trials, especially for Alzheimer’s, Parkinson’s, multiple sclerosis, and epilepsy, are conducted in the U.S., supported by the FDA’s streamlined fast-track and breakthrough therapy designations.
3) Innovation in Trial Design: The U.S. market is adopting adaptive trial designs, AI-based trial management systems, and decentralized trials faster than most regions.
5) Strong Industry-Academic Collaborations: Institutions like NIH, Mayo Clinic, and major pharmaceutical companies collaborate extensively, driving neurological research and precision-medicine-based trials.
Also Read@ Why the U.S. is Setting the Pace for Clinical Research Innovation
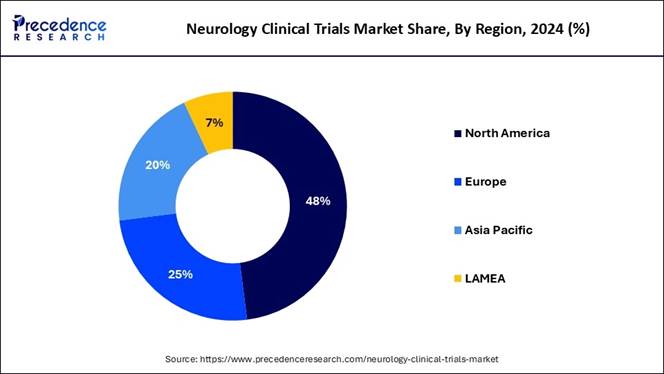
Asia Pacific Emerges as a Hub for Cost-Effective Clinical Trials
Asia Pacific is expected to expand notably during the forecast period, owing to a rapid healthcare infrastructure upgrade and a rise in clinical trial facilities in several regions. Moreover, the regional countries such as India, Japan, and China have seen providing the low-cost trial facilities with faster patient recruitment in recent years, as per the regional survey. Also, the government's push for better healthcare facilities is expected to create lucrative opportunities in the upcoming years.
Key Drivers of India Neurology Clinical Trials Market:
1) Rapid Growth & Emerging Hub: India is emerging as a key destination for neurology clinical trials due to its large patient pool, lower operational costs, and growing clinical research capabilities.
2) Increased Government Support: Government initiatives and revised clinical trial regulations are improving transparency and encouraging global sponsors to conduct trials in India.
3) Growing Infrastructure & Talent Pool: India is witnessing an expansion in neurology research centers, CROs, and trained medical professionals with trial experience, enhancing trial capacity.
4) Focus on Cost-Effective Research: India’s affordability makes it a strategic location for conducting Phase I to III trials, especially for neurodegenerative and rare neurological diseases.
Read Also@ AI in Clinical Trials Market Driving a New Era of Data-Driven Drug Development
Neurology Clinical Trials Market Segmentation Analysis:
By Type Analysis:
Why Did the Phase 2 Segment Dominate the Market in 2024?
The phase 2 segment held the largest share of the neurology clinical trials market in 2024 due to it being considered an important step in the clinical trial, which shows the effectiveness of the treatment in patients with specific neurological diseases. Furthermore, several drugs can fail here, but these tests can filter out the ineffective therapies.
The phase 3 segment is expected to grow at a significant rate, akin to the importance of this phase. This phase is known as the most important step after the 2nd phase, where the safety and effectiveness of the treatment will be confirmed. Furthermore, these tests involved a large number of testing patient groups per the survey.
By Indication Analysis:
How the Epilepsy Segment Maintains Its Dominance in the Current Industry?
The epilepsy segment held the largest share of the market in 2024 because it is one of the most common neurological disorders worldwide, affecting millions of patients. The high prevalence creates a strong demand for new and effective treatments. Clinical trials in epilepsy often focus on developing safer and more targeted anti-seizure drugs with fewer side effects.
The Huntington’s disease segment is expected to grow at a significant rate because of the rising focus on rare neurological disorders that currently have very limited treatment options. Although less common, Huntington's is a severe genetic condition that attracts significant research funding from governments and pharmaceutical companies.
By Study Design Analysis:
Why Did the Interventional Segment Dominate the Market in 2024?
The interventional segment dominated the market with the largest share in 2024, because most neurology clinical trials test the safety and effectiveness of new drugs, therapies, or medical devices directly on patients. Interventional studies are preferred as they provide controlled conditions, measurable outcomes, and clear results on how treatments work.
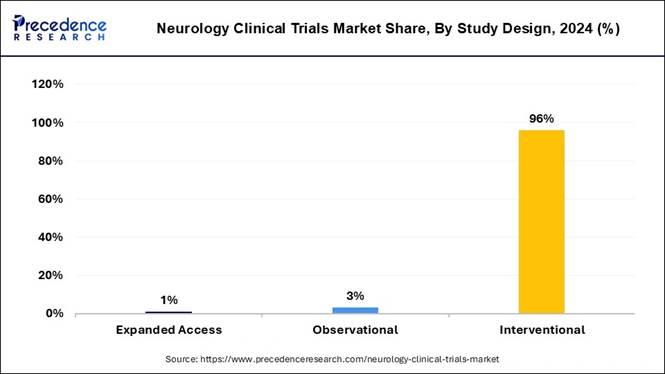
The observational segment is expected to grow at a significant rate because it provides valuable real-world data on how neurological diseases progress and how patients respond to long-term treatments. Unlike interventional trials, observational studies do not alter patient care but monitor outcomes over time, which is important for chronic conditions like Alzheimer's or multiple sclerosis.
Neurology Clinical Trials Market Top Companies:
➢ IQVIA
➢ Biogen
➢ Aurora Healthcare
➢ GlaxoSmithKline Plc.
➢ Icon Plc.
➢ Syneous Health
➢ Charles River Laboratories
➢ Med pace
➢ Covance
➢ Novartis AG
➢ Sanofi
➢ Merck & Co., Inc.
➢ AbbVie Inc.
➢ Teva Pharmaceutical Industries Ltd.
➢ Annovis Bio
➢ Athira Pharma, Inc.
➢ Zydus Group
➢ Eli Lilly and Company
➢ Eisai Co., Ltd.
➢ AstraZeneca
➢ Supernus Pharmaceuticals, Inc. (Adamas Pharmaceuticals)
What is Going Around the Globe?
• In February 2024, a phase I/ phase II clinical trial investigating an allogenic neural exosome AB126 derived from neural stem cells was launched by a biotechnology company, Aruna Bio (GA, US), for the treatment of neurogenerative diseases.
• In March 2024, an innovative and new pTau217 blood test, ALZpath Dx, was launched by a specialized clinical laboratory, Neurocode USA, Inc., that offers world-class testing solutions for neurological disorders. This new test may be used in the monitoring, screening, and diagnosis of Alzheimer's disease. In the US, Neurocode is the first laboratory to make this test an LDT (laboratory-developed test) for clinical trials, clinical diagnostics use, and other research causes.
Neurology Clinical Trials Market Segmentation:
By Phase
• Phase I
• Phase II
• Phase III
• Phase IV
By Study Design
• Interventional
• Observational
• Expanded Access
By Indication
• Epilepsy
• Parkinson’s Disease (PD)
• Huntington’s Disease
• Stroke
• Traumatic Brain Injury (TBI)
• Amyotrophic Lateral Sclerosis (ALS)
• Muscle Regeneration
• Others
By Geography
• North America
• Europe
• Asia-Pacific
• Latin America
• Middle East and Africa
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or Asia Pacific.
Don’t Miss Out! | Instant Access to This Exclusive Report 👉 https://www.precedenceresearch.com/checkout/4657
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344
Stay Ahead with Precedence Research Subscriptions
Unlock exclusive access to powerful market intelligence, real-time data, and forward-looking insights, tailored to your business. From trend tracking to competitive analysis, our subscription plans keep you informed, agile, and ahead of the curve.
Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription
About Us
Precedence Research is a global market intelligence and consulting powerhouse, dedicated to unlocking deep strategic insights that drive innovation and transformation. With a laser focus on the dynamic world of life sciences, we specialize in decoding the complexities of cell and gene therapy, drug development, and oncology markets, helping our clients stay ahead in some of the most cutting-edge and high-stakes domains in healthcare. Our expertise spans across the biotech and pharmaceutical ecosystem, serving innovators, investors, and institutions that are redefining what’s possible in regenerative medicine, cancer care, precision therapeutics, and beyond.
Web: https://www.precedenceresearch.com
Our Trusted Data Partners:
Towards Healthcare | Nova One Advisor | Market Stats Insight
Get Recent News 👉 https://www.precedenceresearch.com/news
For Latest Update Follow Us:
✚ Related Topics You May Find Useful:
💡 Digital Health
in Neurology Market: Explore how AI, wearables,
and remote platforms are reshaping neurological care
💡 Clinical Trial
Imaging Market: Understand the role of
imaging technologies in boosting accuracy and trial outcomes
💡 Medical Device
Clinical Trials Market:
Track how innovation and regulation shape device testing worldwide
💡 Neurogenomics
Market: Discover how genomic
insights are unlocking personalized neurological therapies
💡 Central Nervous
System Therapeutic Market:
Analyze new treatments driving growth in CNS disorder management
💡 Clinical Trial
Materials Manufacturing Market: See how supply efficiency and
compliance are shaping material production
💡 eClinical
Solutions Market: Explore how digital
platforms streamline trial design, data, and reporting
💡 Biopharma
Clinical Trials Market:
Gain insights into how biopharma R&D pipelines accelerate global drug
development
💡 Clinical Trials
Support Services Market:
Examine the growing ecosystem of CROs, logistics, and trial management
providers
💡 Oncology
Clinical Trials Market:
Track how targeted therapies and immuno-oncology fuel cancer research
💡 Clinical Trial
Investigative Site Network Market: Learn how global site networks
improve efficiency and patient recruitment
💡 Clinical Trial
Supplies Market: Explore supply chain
solutions ensuring smooth and compliant trial operations
💡 Clinical Trial
Patient Recruitment Services Market: Understand how digital outreach and
diversity initiatives boost trial enrolment
💡 Mental Health
Clinical Trials Market:
Analyze rising investment in trials for depression, anxiety, and psychiatric
care
💡 Cell and Gene
Therapy Clinical Trials Market: See how advanced therapies are
transforming trial design and patient outcomes

