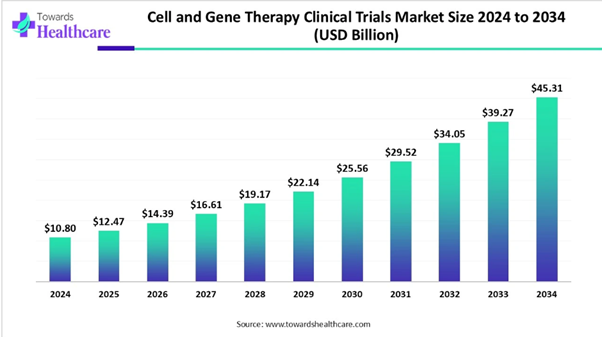
According to Towards Healthcare research, the global cell and gene therapy clinical trials market size is calculated at USD 10.8 billion in 2024, grew to USD 12.47 billion in 2025, and is projected to reach around USD 45.31 billion by 2034. The market is expanding at a CAGR of 15.43% between 2025 and 2034. The global cell and gene therapy clinical trials market is driven by the expanding industries and growing innovations.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5967
Key Takeaways
★ Cell and gene therapy clinical trials market to crossed USD 10.8 billion by 2024.
★ Market projected at USD 45.31 billion by 2034.
★ CAGR of 15.43% expected in between 2025 to 2034.
★ North America, especially the U.S., held the major revenue share of approximately 48% in the cell and gene therapy clinical trials market in 2024.
★ Asia-Pacific is expected to witness the fastest growth during the predicted timeframe.
★ By therapy type, the gene therapy trials segment led the market in 2024 with a share of approximately 52%.
★ By therapy type, the combined cell + gene therapy segment is expected to witness the fastest growth during the forecast period.
★ By phase of trial, the phase I segment dominated the market by number of trials in 2024, with a share of approximately 46%.
★ By phase of trial, the phase III segment is expected to witness the fastest growth in funding volume during the forecast period.
★ By indication, the oncology segment led the market in 2024 with a share of approximately 56%.
★ By indication, the rare genetic disorders segment is expected to witness the fastest growth during the forecast period.
★ By vector type, the AAV (adeno-associated virus) segment led the market in 2024 with a share of approximately 42%.
★ By vector type, the CRISPR/Cas9 & other gene editing platforms segment is expected to witness the fastest growth during the forecast period.
★ By sponsor type, the biotechnology companies segment led the market in 2024 with a share of approximately 58%.
★ By sponsor type, the contract research organizations (CROs) segment is expected to witness the fastest growth by facilitators during the forecast period.
Key Metrics and Overview
|
Table |
Scope |
|
Market Size in 2025 |
USD 12.47 Billion |
|
Projected Market Size in 2034 |
USD 45.31 Billion |
|
CAGR (2025 - 2034) |
15.43% |
|
Leading Region |
North America by 48% |
|
Market Segmentation |
By Therapy Type, By Phase of Trial, By Indication, By Vector Type, By Sponsor Type, By Region |
|
Top Key Players |
Novartis AG, Gilead Sciences, Bristol Myers Squibb, Bluebird Bio/2seventy bio, Sarepta Therapeutics, Sana Biotechnology, CRISPR Therapeutics, Intellia Therapeutics, Editas Medicine, REGENXBIO Inc., Orchard Therapeutics, Beam Therapeutics, Verve Therapeutics, Allogene Therapeutics, Poseida Therapeutics, Rocket Pharmaceuticals, Astellas Gene Therapies, Passage Bio, MeiraGTx |
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What are the Cell and Gene Therapy Clinical Trials?
The cell and gene therapy clinical trials market is driven by growing R&D investments and a supportive regulatory environment. Cell and gene therapy clinical trials refer to the research studies that are conducted to test the efficacy, safety, and long-term effects of cell and gene therapies. These therapies are used to manage, treat, and potentially cure diseases like cancer, rare genetic disorders, neurological disorders, autoimmune diseases, and many others.
What are the Major Growth Drivers in the Market?
A growing pipeline is the major driver in the cell and gene therapy clinical trials market. The growing startups and expanding companies are developing a wide range of cell and gene therapies for various diseases, which is driving their clinical trials. Additionally, growing unmet medical needs, demand for personalized medicines, early diagnosis, and technological advancements are other market drivers.
What are the Key Drifts in the Market?
The cell and gene therapy clinical trials market has been expanding due to the growing funding and collaborations to advance and launch various cell and gene therapy clinical trials.
• In November 2025, a total of $115 million Series C round was secured by Aspen Neuroscience, which will be utilized to advance the clinical progress of ANPD001, which is an investigational autologous induced pluripotent stem cell (iPSC)-derived therapy.
• In November 2025, to develop an indigenous CRISPR-based gene-editing therapy for Sickle Cell Disease (SCD), a technology transfer agreement was signed between the Council for Scientific and Industrial Research’s Institute of Genomic and Integrative Biology (CSIR-IGIB) and Serum Institute of India (SII).
What is the Significant Challenge in the Market?
High cost acts as a significant challenge in the cell and gene therapy clinical trials market. The cost associated with their manufacturing and quality control is high, which makes their clinical trials expensive. Moreover, safety concerns, limited rare disease patients, and regulatory hurdles are some of the other market limitations.
Regional Analysis
Why did North America Dominate the Market in 2024?
In 2024, North America captured the biggest revenue share of the cell and gene therapy clinical trials market, due to the presence of robust biotechnology and pharmaceutical industries. The growth in the healthcare investments is also increasing their development, where the early adoption of advanced technologies has increased their development, leading to clinical trials. Moreover, the institutions also contributed to the development of new therapies, which promoted the market growth.
The U.S. has seen a marked rise in clinical trials for cell and gene therapies driven by growing interest in oncology, rare diseases, and neurodegenerative conditions under supportive regulatory pathways and substantial R&D funding. The expansion of Phase I through Phase III trials reflects increasing confidence in evaluating the safety, dosing, and efficacy of advanced biologic therapies, supporting a deeper clinical research pipeline across diverse therapeutic areas.
What Made the Asia Pacific Grow Notably in the Market in 2024?
Asia Pacific is expected to host the fastest-growing cell and gene therapy clinical trials market during the forecast period, due to expanding industries, which are increasing the R&D activities, driving the development of novel cell and gene therapies. The presence of large patient volume and government support is also accelerating their clinical trials. Moreover, the growing CRO and adoption of advanced technologies are increasing their development of new therapies and supporting the clinical trials, promoting the market growth.
In China, the landscape of cell and gene therapy trials has accelerated sharply, with recent years registering large increases in new trials and first conditional approvals for stem-cell-based treatments under national regulatory endorsement. Chinese developers are increasingly exploring CAR-T therapies, regenerative medicine, and expanding into solid tumors, autoimmune disorders, and gene-based therapies, signaling a strategic shift from narrow early-phase research to broader clinical applications.
Segmental Insights
By therapy type analysis
Why Did the Gene Therapy Trials Segment Dominate in the Market in 2024?
By therapy type, the gene therapy trials segment dominated the cell and gene therapy clinical trials market in 2024, due to high unmet needs. This increased the demand for curative and one-time treatment options. The investment also supported the development of various gene therapies, which promoted their trials.
By therapy type, the combined cell + gene therapy segment is expected to show the highest growth during the upcoming years, driven by its synergistic effect. This is an encouraging development of new combination therapies, which are being backed by investment and funding. Additionally, the growing rare diseases is increasing their use.
By Phase of Trial analysis
Which Phase of Trial Type Segment Held the Dominating Share of the Market in 2024?
By phase of trial, the phase I segment held the dominating share of the cell and gene therapy clinical trials market in 2024, due to growth in innovations. Additionally, the ongoing development and expanding pipelines also promoted the phase I clinical trials. Furthermore, their extensive safety profile assessment is conducted in this phase.
By phase of trial, the phase III segment is expected to show the fastest growth rate in funding volume during the upcoming years, driven by the growing success rates and candidates. Moreover, the utilization of advanced platforms is accelerating the pipeline development, which is driving the trials.
By indication analysis
What Made Oncology the Dominant Segment in the Market in 2024?
By indication, the oncology segment dominated the cell and gene therapy clinical trials market in 2024, due to the growth in cancer incidences. This is increasing the cell and gene therapies development, due to their growing adoption. The industries and institutes also contributed to their innovation, increasing their clinical trials.
By indication, the rare genetic disorders segment is expected to show the highest growth during the forthcoming years, driven by the growing success rates. The adoption of cell and gene therapies are also being driven by increasing demand for effective treatment options, where the funding and adoption of advanced technologies are promoting their development and trials.
By vector type analysis
How the AAV (Adeno-Associated Virus) Segment Dominated the Market in 2024?
By vector type, the AAV (adeno-associated virus) segment held the largest share of the cell and gene therapy clinical trials market in 2024, due to its enhanced safety and low immune response. This increased their use for treatment of chronic disorders, where the growth in their acceptance encouraged innovation, driving their trials.
By vector type, the CRISPR/Cas9 & other gene editing platforms segment is expected to show the fastest growth rate during the predicted time, driven by their high efficiency. Moreover, the growing investment and technological advancements are increasing their accuracy and applications, leading to new clinical trials.
By sponsor type analysis
Why Did the Biotechnology Companies Segment Dominate in the Market in 2024?
By sponsor type, the biotechnology companies segment dominated the cell and gene therapy clinical trials market in 2024, driven by growth in innovations. The expanding R&D infrastructure and interest in niche areas increased the development of cell and gene therapies for wide range of applications, which led to their clinical trials supported by investments.
By sponsor type, the contract research organizations (CROs) segment is expected to show the highest growth by facilitators during the predicted time, driven by growing outsourcing trends. Furthermore, the growing operation and regulatory complexities are also increasing the collaborations to accelerate the cell and gene therapy development and clinical trials.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Recent Developments in the Market
• In December 2025, the successful dosing of the first patient for the Phase 1/2 clinical trial of EN-374, which is an in vivo hematopoietic stem cell (HSC)-directed gene insertion therapy for X-linked chronic granulomatous disease (X-CGD) treatment, was announced by Ensoma.
• In December 2025, the global phase II clinical trial of the novel TYK2 inhibitor, Soficitinib (ICP-332), for the treatment of patients with prurigo nodularis was announced to have been initiated by dosing of the first patient in China, which was announced by InnoCarePharma
• In November 2025, the data of the Acclaim-1 Phase 1 clinical trial of Reqorsa® Gene Therapy in combination with Tagrisso® for the treatment of advanced non-small cell lung cancer (NSCLC) was released by Genprex, Inc. in the Clinical Lung Cancer Journal.
Cell And Gene Therapy Clinical Trials Market Key Players List

• Bristol Myers Squibb
• Novartis AG
• Bluebird Bio/2seventy bio
• Gilead Sciences
• CRISPR Therapeutics
• Sarepta Therapeutics
• Intellia Therapeutics
• REGENXBIO Inc.
• Sana Biotechnology
• Orchard Therapeutics
• Poseida Therapeutics
• Editas Medicine
• Allogene Therapeutics
• Astellas Gene Therapies
• Beam Therapeutics
• MeiraGTx
• Verve Therapeutics
• Rocket Pharmaceuticals
• Passage Bio
Segments Covered in The Report
By Therapy Type
• Gene Therapy Trials
o In vivo (AAV, lentiviral vectors, CRISPR)
o Ex vivo gene-modified cells
• Cell Therapy Trials
O CAR-T, TCR-T, NK cell therapies
• Combined Cell + Gene Therapy
O Engineered cell therapies with gene editing
• RNA-Based Therapies
O mRNA, antisense oligonucleotides
By Phase of Trial
• Phase I
• Phase I/II
• Phase II
• Phase III
• Phase IV/Long-Term Follow-Up Studies
By Indication
• Oncology
O Hematologic cancers (NHL, MM, ALL)
O Solid tumors (glioblastoma, melanoma, lung)
• Rare Genetic Disorders
• Hemophilia, SMA, DMD, SCID
• Ophthalmology
• Retinal dystrophies (e.g., LCA)
• Neurology
• Parkinson’s, Huntington’s, ALS
• Infectious & Autoimmune Diseases
• HIV, Lupus, Type 1 Diabetes (emerging pipeline)
By Vector Type
• AAV (Adeno-Associated Virus)
• Lentiviral Vectors
• CRISPR/Cas9 & Other Gene Editing Platforms
• Retroviral Vectors
• mRNA/LNP Systems
• Non-Viral Delivery (e.g., electroporation, nanoparticles)
By Sponsor Type
• Biotechnology Companies
• Large Pharmaceutical Companies
• Academic Medical Centers
• Nonprofits & Government Institutions
• Contract Research Organizations (CROs)
By Region
• North America
• South America
• Europe
• Asia Pacific
• MEA
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/5967
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

