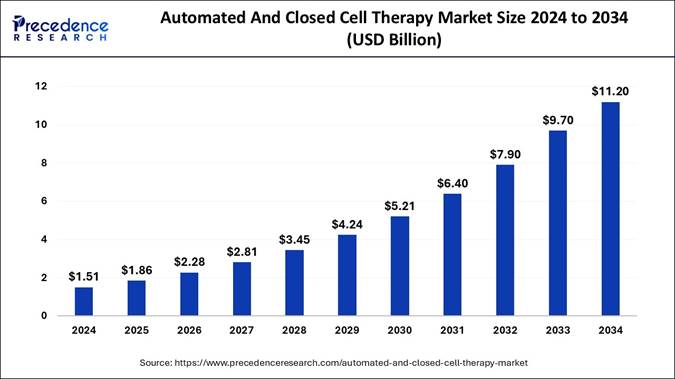
The global automated and closed cell therapy market size is expected to be worth USD around USD 11.20 billion by 203 increasing from USD 1.86 billion in 2025. The North American market achieved the highest market share in 2024, accounting for 46.24%. The market continues to grow rapidly because of technological advancements and the rising need for individualized therapies in regenerative medicine.
According to Precedence Research, the automated and closed cell therapy market size is valued at USD 1.86 billion in 2025 and is predicted to grow from USD 2.28 billion in 2026 to USD 11.20 billion by 2034. The market is expected to expand at a healthy CAGR of 22.19% from 2025 to 2034.
The Full Study is Readily Available | Download the Sample Pages of this Report@
https://www.precedenceresearch.com/sample/2758
📈 Automated and Closed Cell Therapy Market Highlights:
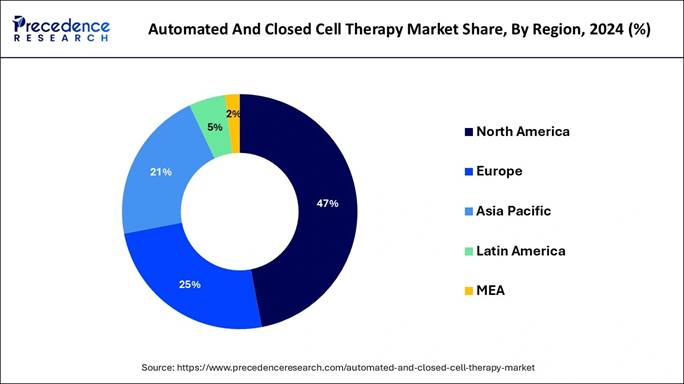
→ North America led the market in 2024, capturing the maximum share of 46.24%.
→ The Expansion segment dominated the market by Workflow.
→ Stem Cell Therapy was the largest segment by Type.
→ The Pre-commercial/R&D Scale segment was the largest by Scale, accounting for 73% of the market share.
Automated and Closed Cell Therapy Market Overview and Industry Potential
The automated and closed cell therapy market develops innovative technologies for more efficient and secure production of cell-based therapies. Automated critical procedures like cell isolation, expansion, and cell cryopreservation operate within these systems to enhance process consistency and reduce the chances of contamination during manufacturing. These advanced technologies will assume essential roles because personalized medicine and regenerative medicine is experiencing growing demand.
This market contains automated and closed systems that serve as essential platforms for developing high-quality therapeutic cells like stem cells and immune cells for medical uses. The automated and closed-cell therapy processing systems market is experiencing rapid expansion through the rising acceptance of therapeutic medicines and cellular therapy applications. The increasing demands for personal treatment methods have fostered increased use of body cell-based therapies such as stem cell treatment, genetic modification, and immune therapy applications.
➡️ Become a valued research partner with us ☎ https://www.precedenceresearch.com/schedule-meeting
Automated and Closed Cell Therapy Market Major Trends
Automation in Cell Processing
Cell therapy manufacturing benefits from automation as it enables efficiency improvement and scalable manufacturing procedures. Production quality grows better through automated systems while they simultaneously cut human errors and minimize processing times. Large-scale production requires this trend because it allows increased production speed and consistent outcomes, which serve to fulfill rising cell therapy requirements.
Technological Advancements
The implementations of these new technologies lead to increased accuracy and enhanced cell preservation without raising contamination potential. The development of modern technologies enables the fulfillment of strict standards in cell therapy manufacturing, thus expanding the automated and closed cell therapy market sector.
Regulatory Support and Approvals
The government and regulatory authorities are offering supportive policies and rapid cell therapy approval methods to facilitate their advancement. The regulatory developments cut down new therapy commercialization timelines, thereby prompting enhanced research initiatives as well as production investments. Regulatory approval serves as an essential enabler for cell therapy production facilities to adopt automatic procedures because it maintains industry standards.
📥 See the Full Analysis Here 👉 https://www.precedenceresearch.com/automated-and-closed-cell-therapy-market
AI Integration in the Automated and Closed Cell Therapy Market
Artificial intelligence (AI) serves as a fundamental component for bringing automation to the systems that handle cell therapy processing. This technology enables improved accuracy by lowering operator mistakes, so it speeds up the production of cell therapies. Manufacturing failure rates decrease through automation because checkpoint analysis becomes possible and human errors are diminished.
AI supports cell therapy manufacturing across different production scales, starting at the laboratory and progressing through pilot and commercial levels for consistent, high-quality outcomes. AI revolutionizes aspects of cell manufacturing, starting from cell separation up to activation and genetic modification, as well as cell expansion.
Automated and Closed Cell Therapy Market Report Scope
|
Report Attributes |
Key Details |
|
Market Size in 2025 |
USD 1.86 Billion |
|
Market Size by 2034 |
USD 11.20 Billion |
|
Growth Rate (2025–2034 CAGR) |
22.19% |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2034 |
|
Largest Market |
North America |
|
Regions Covered |
North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
|
Segments Covered |
Workflow, Type, and Scale |
|
Key Takeaways |
• North America accounted
for approximately 46.24% of total revenue in 2024. |
|
Market Definition |
The market includes automated devices and closed-system equipment used for manufacturing, processing, and delivery of cell-based therapies, improving standardization, scalability, and reducing contamination risks. |
|
Market Drivers |
Growing burden of chronic and degenerative diseases, rising demand for personalized medicine, and increasing adoption of advanced automated bioprocessing technologies. |
|
Market Restraints |
High cost of manufacturing and equipment, strict regulatory frameworks, and limited access to skilled professionals. |
|
Market Opportunities |
Expanding regenerative medicine applications, aging global population, rapid research in stem cell and CAR-T therapy production, and increased investment by biotech companies. |
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/2758
🌐 CASE STUDY: The Pivotal Shift to Scalable, Intelligent Cell Manufacturing
The Automated and Closed Cell Therapy Market is undergoing a fundamental transformation, moving from manual, small-scale lab procedures to robust, scalable, and contamination-free industrial manufacturing. This shift is best illustrated by the strategic collaborations between automation technology specialists and major cell therapy developers.

Scenario: The Challenge of Commercializing Cell Therapies
The increasing success of cell therapies (especially the largest segment, Stem Cell Therapy) and the rapid growth in Pre-commercial/R&D scale activity (accounting for 73% of the market) created an urgent bottleneck. Traditional manual processing for critical steps like Expansion (the largest segment by Workflow) is expensive, slow, inconsistent, and highly prone to contamination.
The Solution: Strategic Partnerships & End-to-End Automation
Top
market players, including Thermo Fisher Scientific Inc., Fresenius Kabi AG,
Lonza, and Sartorius AG, are tackling this by partnering to create
fully integrated, closed-loop systems.
|
Key Collaboration |
Date |
Technology Integration |
Impact on Market Growth |
|
Multiply Labs & Thermo Fisher Scientific Inc. |
February 2024 |
Focused on automating core manufacturing operations: cell expansion and separation. |
Demonstrates the integration of advanced robotics with industry-standard instruments to achieve high-speed, repeatable, and scalable production of therapeutic cells. |
|
Cellular Origins & Fresenius Kabi AG |
October 2024 |
Developed automated solutions to enhance production output and system scalability. |
Aims for 24/7 fully automated, clinical-grade manufacturing, significantly reducing manual labor and human error, which are essential steps for cost-effective commercialization. |
|
Sino-Biocan (Product Launch) |
October 2024 |
Released the WUKONG Automated, Closed, and Integrated Cell Processing System. |
Represents the emergence of fully autonomous, closed-loop platforms that handle the entire process, from cell collection to final drug production, minimizing contamination risks. |
The Critical Role of AI
The next layer of efficiency is Artificial Intelligence (AI). AI acts as the process intelligence, enabling these automated systems to:
🔸Optimize Yield and Quality: Analyze vast production data to predict optimal cell growth conditions and detect quality deviations in real-time.
🔸Reduce Batch Failure: Lower manufacturing failure rates by performing real-time checkpoint analysis and mitigating human errors, ensuring consistent, high-quality outcomes at commercial scale.
Conclusion
The market's predicted growth to USD 11.20 billion by 2034, up from USD 1.86 billion in 2025 is directly enabled by this shift. The integration of closed systems, robotic automation, and AI transforms cell therapy from a lab-based curiosity into a reliable, scalable, and accessible treatment, driven by the increasing need for individualized regenerative medicines.
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Automated and Closed Cell Therapy Market Key Regions
North America Automated and Closed Cell Therapy Market Trends:
North America will lead the global automated and closed cell therapy market in 2024. The established healthcare facilities and leading research structures in this region serve as essential components for its market dominance.
The research and development investments for regenerative medicines keep rising at the same time as clinical trials increase. Market expansion receives support from the existing robust healthcare infrastructure and continuing investments between players and research institutes that enhance growth prospects.
What is the U.S. Automated and Closed Cell Therapy Market Size?
The U.S. automated and closed cell therapy market size is estimated at USD 610 million in 2025 and is projected to reach nearly USD 3,920 million by 2034, expanding at a notable CAGR of 23.11% from 2025 to 2034.
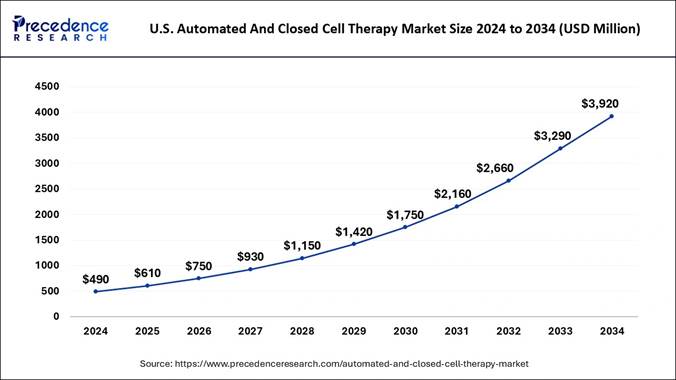
United States Automated and Closed Cell Therapy Market Trends:
The United States maintained its dominance in the automated and closed cell therapy market due to the presence of the world's strongest and advanced healthcare systems and biotech research. Moreover, several major brands are actively investing in cell and gene therapy while researching safer and faster cell therapy in recent years. Also, the clear regulatory framework, where agencies like the FDA and others are supporting the industry's potential in the current period.
Note: This report is readily
available for immediate delivery. We can review it with you in a meeting to
ensure data reliability and quality for decision-making.
📥 Download Sample Pages for
Informed Decision-Making 👉 https://www.precedenceresearch.com/sample/2758
Asia Pacific Automated and Closed Cell Therapy Market Trends:
Asia Pacific is expected to witness lucrative growth in the automated and closed cell therapy market during the forecast period. Companies are increasing investments in automation technologies that improve manufacturing efficiency as well as scalability and precision during advanced cell and gene therapy production. This capital expenditure serves to fulfill the increasing demand for high-quality treatments while improving production processes. Regulatory organizations have made new therapy approval processes more expedited, through which innovative treatments can access the market faster.
🔸In July 2024, Bioserve India introduced its sophisticated stem cell products to the Indian market. The new scientific offerings from REPROCELL seek to enable research-driven innovation, which boosts drug development and regenerative medicine progress in the Indian Market.
China Automated and Closed Cell Therapy Market Trends:
China is expected to emerge as a prominent country for the automated and closed-cell therapy market in the coming years, owing to the country's rapid investment in biotechnology and healthcare advancements. China has advantageous factors than the whole world, such as skilled scientists, early access to advanced technology, and cheap labor are likely to create significant opportunities for the region during the forecast period.
Automated and Closed Cell Therapy Market Segmentation:
Workflow Insights
The expansion segment held the highest share of the automated and closed-cell therapy market in 2024. Cell expansion stands as a fundamental procedure during the manufacturing process of cells. The automated cell expansion market is experiencing growth due to the superior advantages that automated processes provide over traditional manual activities. The automation process improves the speed of procedures and ensures high levels of reproducibility. This segment expands because healthcare markets require scalable production systems with automated and efficient technology to enhance the manufacturing quality and potency of cell and gene therapies, especially when treating complex solid tumors.
The separation segment is anticipated to grow rapidly over the forecast period. The increasing adoption of automated systems for cell separation has led to better efficiency because these systems perform gentle sample handling and minimize contamination risks. Higher cell therapy production requirements regarding scalability and efficiency have led manufacturers to choose automated and closed-cell separation systems. The critical processes in cell therapy manufacturing, which include cell expansion and separation, receive increased efficiency and reduced costs because of automation.
🔸In February 2024, Multiply Labs and Thermo Fisher Scientific Inc. developed automated solutions for essential cell therapy manufacturing operations, which centered on cell expansion as well as separation processes.
Type Insights
The stem cell therapy segment held the largest share of the automated and closed cell therapy market. Stem cells contain two specific traits, including the capability to renew themselves along with their potential to generate specialized cells, for instance, muscle cells and cells of blood and nerves. The cells function as key components that enable body tissues to carry out maintenance activities and repair duties. The market growth of stem cell therapies will advance because they successfully treat diseases like leukemia and demonstrate potential for treating multiple conditions.
🔸In October 2024, Cellular Origins and Fresenius Kabi AG established a business partnership to create automated solutions for manufacturing cell and gene therapies. The partnership worked to enhance production output while achieving higher system scalability to support increasing market needs.
The non-stem cell therapy segment is expected to witness a notable growth rate during the forecast period. Medical demand for non-stem cell treatments keeps rising since these cell types provide specialized functions, while researchers can modify them for exact therapeutic purposes. The increasing demand for automated cell processing systems has become prevalent in biological research because they deliver efficient operations combined with reproducible results with high-quality outcomes. The growing number of cancer cases and congenital disorders stimulates the market need for non-stem cell treatments.
Scale Insights
The pre-commercial/R&D scale segment held the largest automated and closed cell therapy market share. This segment encompasses production activities at research and academic centers, innovation hubs, and other R&D facilities. These research centers will experience substantial expansion owing to their increasing investment activities in developing cell therapy research.
Research facilities that work on developing new therapies and conducting clinical trials require automated and closed systems to enhance their production process because their emphasis on medical advancement has driven up such system requirements.
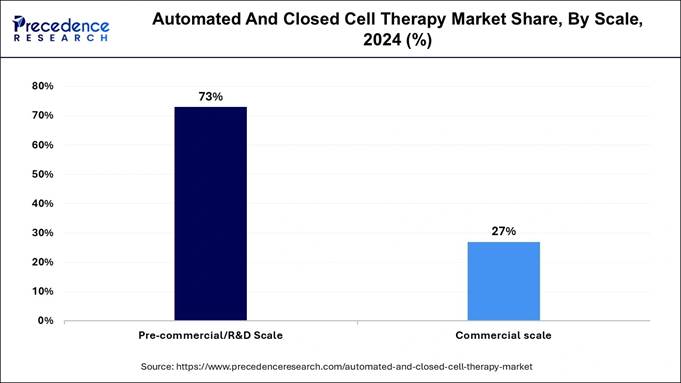
The commercial scale segment is expected to witness a notable growth rate during the forecast period. This segment expands due to the growing commercial acceptance of scalable, high-quality, efficient production solutions made for large-scale cell therapy manufacturing activities. Large-scale manufacturing becomes efficient and economical through automation methods to fulfill the rising commercial cell therapy market needs.
🔸In May 2024, a new partnership between ADVA Biotechnology and Cellipont Bioservices started to improve cell therapy manufacturing through the ADVA-X3 platform. ADVA-Biotechnology formed this collaboration to enhance critical aspects of cell therapy manufacturing, including scalability, efficiency, and quality control.
✚ Related Topics You May Find Useful:
➡️ Automated and Closed Cell Therapy Processing System Market: Explore innovations enhancing scalability, safety, and precision in next-generation cell therapy production
➡️ Automated Cell Counter Market: Learn how automation is transforming lab efficiency, accuracy, and high-throughput sample processing
➡️ Engineered T-Cells Market: Track advances in CAR-T and TCR therapies reshaping oncology treatment outcomes
➡️ Automated Bio-Banking Market: Understand how robotics and AI are improving biospecimen storage, traceability, and long-term research value
➡️ Automated Analyzers Market: Discover how next-generation analyzers enable rapid diagnostics and streamline clinical laboratory workflows
➡️ Closed System Transfer Device Market: Analyze rising demand for safety-optimized drug handling solutions in oncology and hospital pharmacy settings
➡️ Automated Sample Storage Systems Market: See how automation enhances temperature-controlled storage integrity for pharmaceutical and research samples
➡️ Automated Analyzers Market: Examine the role of advanced automation in increasing diagnostic speed, standardization, and productivity worldwide
Automated and Closed Cell Therapy Market Top Companies
➢ Thermo Fisher Scientific Inc.
➢ Miltenyi Biotec GmbH
➢ Sartorius AG
➢ Lonza Group AG
➢ Terumo BCT, Inc.
➢ Fresenius SE & Co. KGaA
➢ GE Healthcare
➢ Pall Corporation
➢ Asahi Kasei Corporation
➢ Merck KGaA
What is Going Around the Globe?
🔸In January 2025, CellFE, the leading non-viral gene editing Technology Company, released its first-in-class CellFE T-Rest (Resting T Cell Kit) manufacturing media product to specifically serve resting T cell procedures.
🔸In December 2024, Thermo Fisher Scientific Inc. launched the Gibco CTS Detachable Dynabeads CD4 and CTS Detachable Dynabeads CD8 (CTS Detachable Dynabeads) as a global leader in science service provision. These newest products continue advancing the CTS Detachable Dynabeads platform from Thermo Fisher as they deliver cell therapy isolation and activation products that prioritize cellular quality and offer enhanced workflow control.
🔸In October 2024, Sino-Biocan released the self-developed WUKONG Automated, Closed, and Integrated Cell Processing System through its existing modular cell preparation platforms. The advanced system provides autonomous operation capabilities through its fully automated, continuously intelligent, and closed-loop mechanisms.
Segments Covered in the Report
By Workflow
🔹 Separation
🔹 Expansion
🔹 Apheresis
🔹 Fill – Finish
🔹 Cryopreservation
🔹 Others
By Type
🔹 Stem Cell Therapy
🔹 Non-Stem Cell Therapy
By Scale
🔹 Pre-commercial/R&D Scale
🔹 Commercial scale
By Region
🔹 North America
🔹 Europe
🔹 Asia Pacific
🔹Latin America
🔹Middle East & Africa (MEA)
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or Asia Pacific.
Don’t Miss Out! | Instant Access to This Exclusive Report 👉
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344
Stay Ahead with Precedence Research Subscriptions
Unlock exclusive access to powerful market intelligence, real-time data, and forward-looking insights, tailored to your business. From trend tracking to competitive analysis, our subscription plans keep you informed, agile, and ahead of the curve.
Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription
About Us
Precedence Research is a global market intelligence and consulting powerhouse, dedicated to unlocking deep strategic insights that drive innovation and transformation. With a laser focus on the dynamic world of life sciences, we specialize in decoding the complexities of cell and gene therapy, drug development, and oncology markets, helping our clients stay ahead in some of the most cutting-edge and high-stakes domains in healthcare. Our expertise spans across the biotech and pharmaceutical ecosystem, serving innovators, investors, and institutions that are redefining what’s possible in regenerative medicine, cancer care, precision therapeutics, and beyond.
Web: https://www.precedenceresearch.com
Our Trusted Data Partners:
Towards Healthcare | Nova One Advisor | Onco Quant | Statifacts
Get Recent News 👉 https://www.precedenceresearch.com/news
For Latest Update Follow Us:
LinkedIn | Medium | Facebook | Twitter
✚ Explore More Market Intelligence from Precedence Research:
➡️ Digital Therapeutics: How software-based interventions are restructuring chronic-disease management and clinical-grade behavioral therapy
➡️ Life Sciences Growth: Forces driving expansion across biotech, biopharma, and advanced therapeutic platforms
➡️ Viral Vector Gene Therapy Manufacturing: Manufacturing constraints, scalability limits, and innovations shaping next-generation gene-delivery systems
➡️ Wellness Transformation: How prevention-centric health models are shifting consumer behavior, product pipelines, and care delivery
➡️ Generative AI in Healthcare: How generative models are unlocking new diagnostics, clinical automation, and patient-care innovations

