Phase II
Recursion’s oral drug candidate for cerebral cavernous malformation showed no improvements in patient- or physician-reported outcomes at 12 months. The biotech will engage with the FDA to determine the need for an additional study.
The highly anticipated results come as the company makes significant changes to its C-suite. Despite the turnover, Dyne said it is looking toward expedited approval pathways for its DMD treatment.
NuCana’s chemotherapy replacement has failed to improve progression-free survival in a Phase II test, sending the biotech’s shares down by 50%.
Neurocrine Biosciences’ potential competitor to Bristol Myers Squibb’s KarXT improved symptoms of schizophrenia in a Phase II trial, but only at the low dose tested.
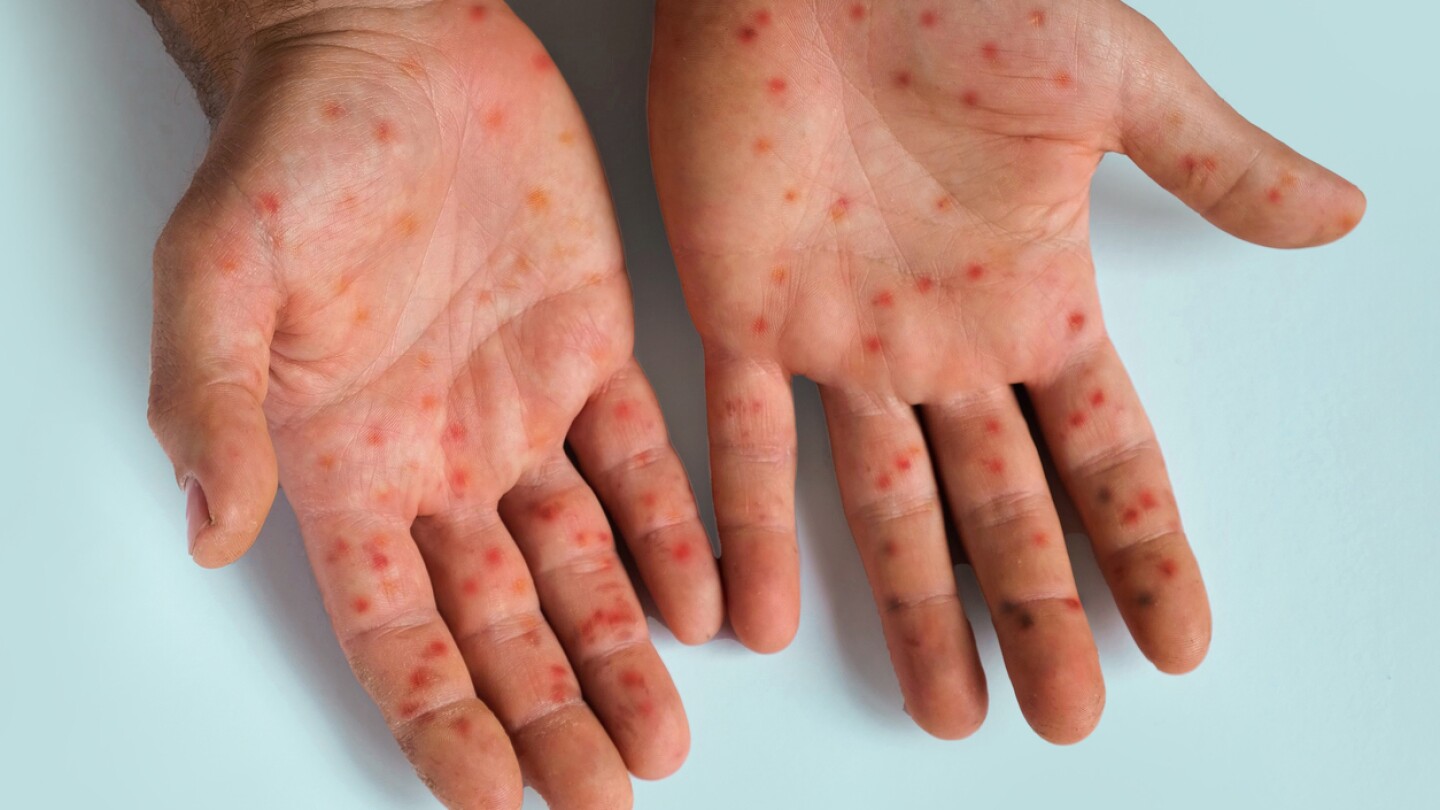
SIGA Technologies’ TPOXX did not outperform placebo at resolving lesions in patients with clade I mpox, the new strain that has spread through parts of Africa and is reaching beyond the continent.
Hundreds of companies are currently running clinical trials in the increasingly lucrative obesity space. BioSpace looks at five candidates with data expected before the end of the year.
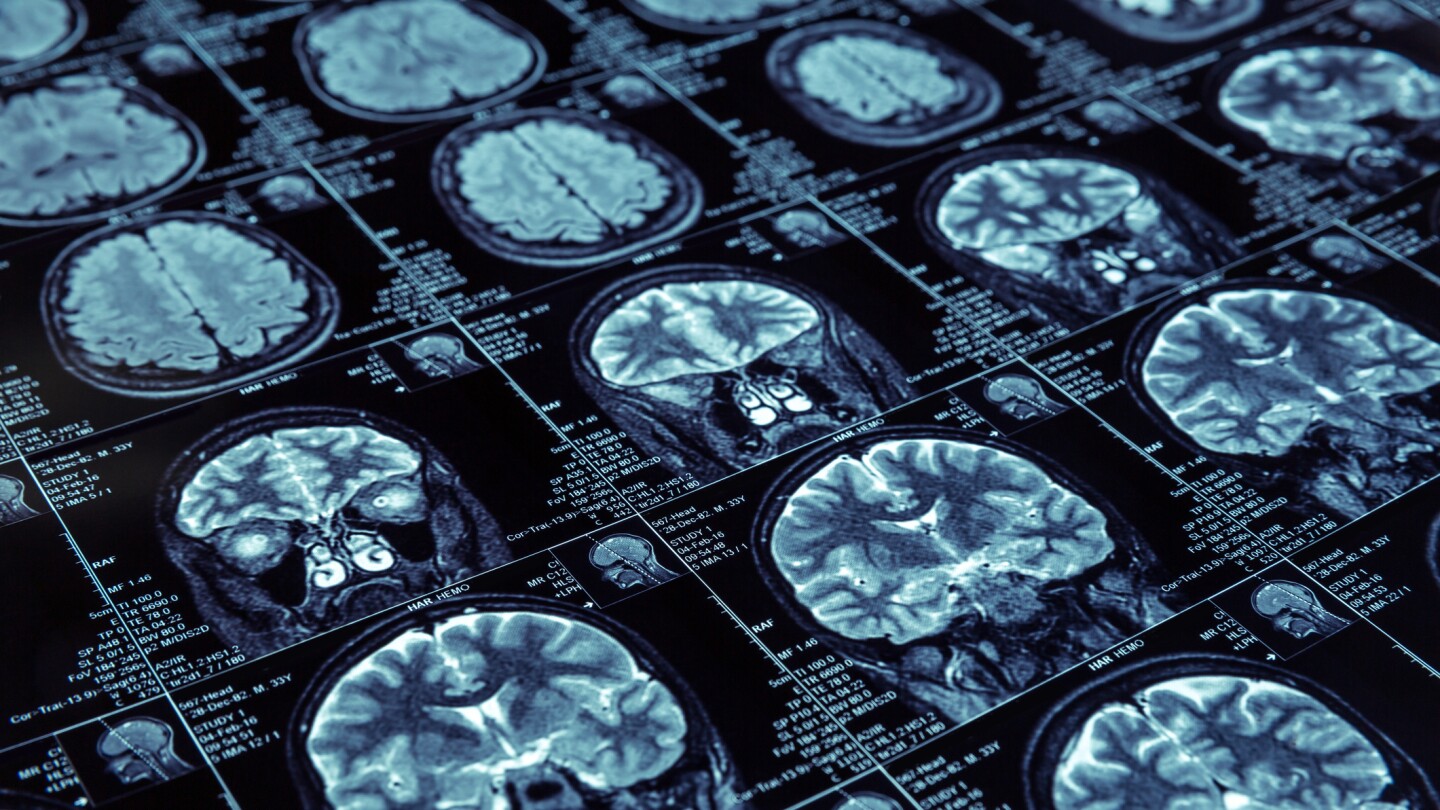
Cognitive function in the liraglutide cohort declined 18% slower than in the placebo arm over one year of treatment, researchers announced Tuesday at the Alzheimer’s Association International Conference.
BioNTech and Regeneron will face off against Merck and Moderna, which are advancing their investigational cancer vaccine mRNA-4157/V940 in combination with Keytruda, in advanced melanoma.
Back-to-back failures in psoriasis and Crohn’s disease have forced Ventyx Biosciences to abandon the development of its investigational oral TYK2 inhibitor VTX958.
With promising Phase II data in hand, Viking Therapeutics is pushing its subcutaneous GLP-1/GIP receptor dual agonist into late-stage development, the company announced on Wednesday.
PRESS RELEASES