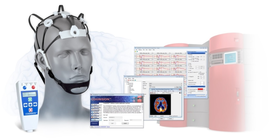
LOUISVILLE, Ky., Nov. 18, 2015 (GLOBE NEWSWIRE) -- Neuronetrix announced today the publication of results from its multi-center clinical trial in a prestigious Alzheimer's journal. The report, "A Clinical Trial to Validate Event-Related Potential Markers of Alzheimer's Disease in Outpatient Settings", is available online now and will be printed in the December issue of Alzheimer's & Dementia: Diagnosis, Assessment and Disease Monitoring, an open access journal of the Alzheimer's Association. This peer-reviewed report validates that ERP biomarkers collected in normal clinical settings can reproduce the results of numerous scientific studies performed in academic research labs.
The study was performed at seven clinical sites in the US and involved a total of 204 test subjects. The 103 mild Alzheimer's patients and 101 age-matched cognitively healthy subjects were each given a brief cognitive test battery, after which the site's clinical staff administered a standardized 25-minute test of brain function using Neuronetrix's COGNISION™ System. This test, called event-related potentials (ERP), records the electrical activity from the brain while the test subject listens through earphones to a sequence of tones. The sequence of tones is designed so that the subject's brain must process the information in a very structured way.
The output of the test is a waveform with a well-defined shape that represents the coordination of cognitive resources and the speed of brain processing. Abnormal changes to the shape of this waveform can reflect cognitive deficits like those experienced by Alzheimer's sufferers.
The results reported in the paper validated the significant differences between healthy individuals and subjects with mild Alzheimer's disease which had been previously reported in smaller studies. The paper goes on to document the abnormal features of the ERP waveform which are useful for identifying the subtle changes that occur in early Alzheimer's disease and describes the specific cognitive functions which are represented by those ERP features.
"This is the first large, multi-center ERP study performed in standard clinical settings where the test administrators were not ERP experts. It was exciting that our results reproduced the sensitivity and robustness of studies performed in state-of-the-art academic labs," said Dr. Marco Cecchi, Director of Clinical Affairs for Neuronetrix.
"With the use of an integrated hardware/software system for ERP testing and automated data analysis tools, Neuronetrix has established a benchmark for the application of ERP biomarkers in Alzheimer's disease research," said Dr. Murali Doraiswamy, a professor at the Duke Institute for Brain Sciences, and a scientific advisor to Neuronetrix. Dr Doraiswamy, a co-author on the publication, added that "The development and validation of noninvasive neural markers to assess cognition and screen for early dementia is a high priority for the field."
About COGNISION™
| |||||
COGNISION™ is an FDA cleared device used by clinicians to perform EEG and event-related potential testing. These tests provide physiological measures of brain processing which can be critical in evaluating a wide range of cognitive disorders.
About Neuronetrix
Neuronetrix is transforming the diagnosis of patients with cognitive disorders by providing accurate and meaningful diagnostic information to health care professionals early in the disease process. This information is critical to clinicians who wish to provide optimal care for their patients and to scientists who are searching for ways to treat these devastating disorders.
A photo accompanying this release is available at:
http://www.globenewswire.com/newsroom/prs/?pkgid=37687
CONTACT: Matt Ullum Neuronetrix 1044 E. Chestnut St. Louisville KY 40204 (502) 561-9040 marketing@neuronetrix.com www.neuronetrix.com
![]()