Eosinophilic Esophagitis Market Outlook 2024-2034:
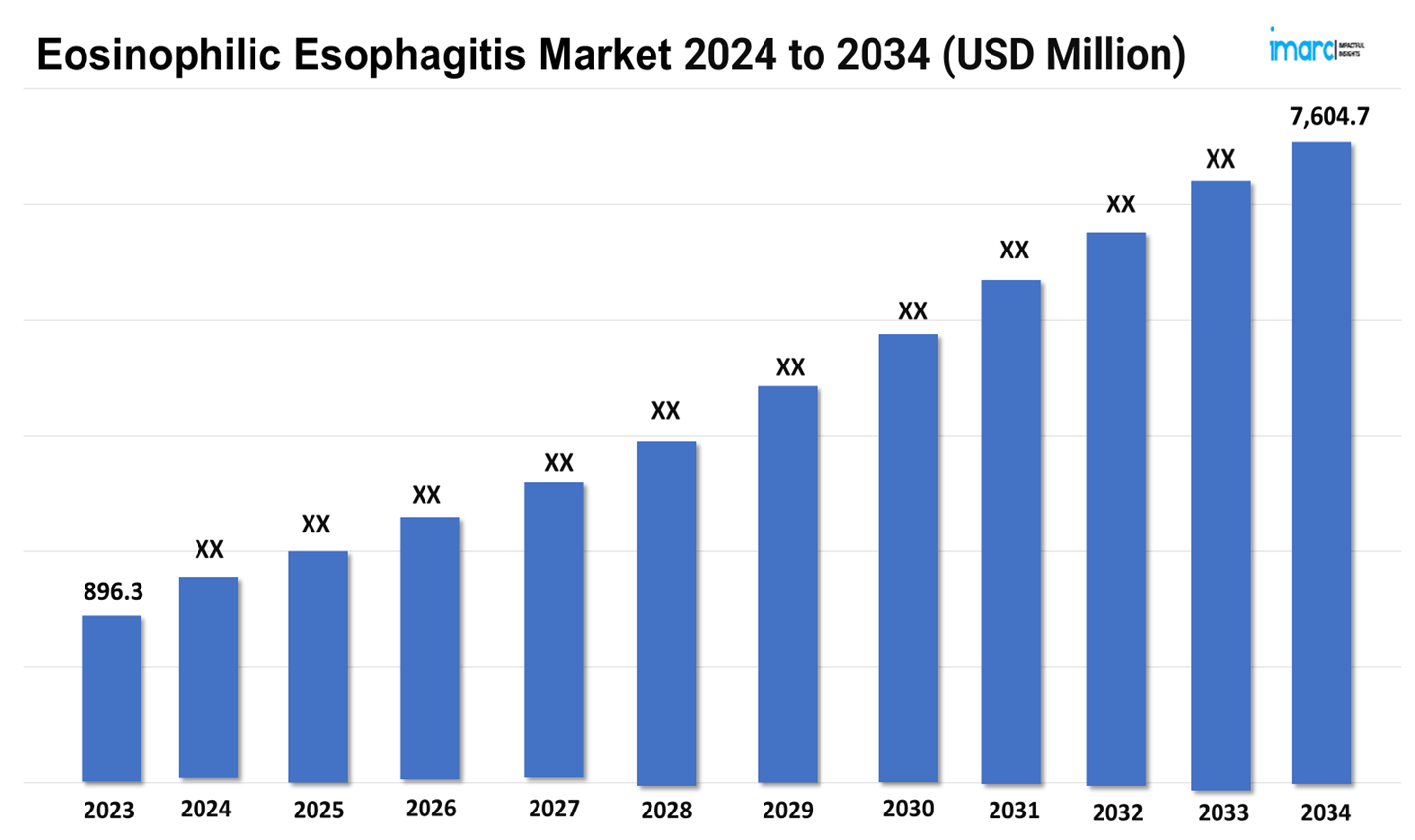
The eosinophilic esophagitis market size reached a value of USD 896.3 Million in 2023. Looking forward, the market is expected to reach USD 7,604.7 Million by 2034, exhibiting a growth rate (CAGR) of 21.46% during 2024-2034. The market is driven by advancements in diagnostic technologies and increased awareness. There is a growing emphasis on personalized treatments, including biologics and novel therapeutic agents. Market growth is also fueled by ongoing research and rising prevalence, with a focus on improving patient outcomes and management strategies.
Advancements in Biologics: Driving the Eosinophilic Esophagitis Market
Recent advancements in biologics are revolutionizing the eosinophilic esophagitis (EoE) market, offering new hope for patients with this chronic and often debilitating condition. Biologics, which are therapeutic agents derived from living organisms, target specific pathways and proteins involved in the inflammatory process of EoE. These treatments have emerged as a significant breakthrough, addressing the limitations of traditional therapies such as corticosteroids and dietary modifications. One of the key advancements is the development of monoclonal antibodies that target specific cytokines involved in the pathogenesis of EoE. For instance, agents like dupilumab, an IL-4/IL-13 inhibitor, have shown promising results in clinical trials by reducing eosinophilic inflammation and improving symptoms. These biologics work by interfering with the immune system's inflammatory responses, thereby providing more targeted and effective treatment options compared to conventional therapies. Another notable advancement is the ongoing research into novel biologics that aim to inhibit eosinophil migration and activation. These therapies focus on the underlying mechanisms driving EoE, such as the overproduction of eosinophils, which are a hallmark of the disease. By addressing these specific pathways, these biologics offer the potential for more durable and sustained relief for patients.
Request a PDF Sample Report: https://www.imarcgroup.com/eosinophilic-esophagitis-market/requestsample
Moreover, the development of these advanced therapies is supported by enhanced diagnostic tools, which allow for more precise identification of patients who are most likely to benefit from biologic treatments. This personalized approach ensures that the right patients receive the most appropriate therapies, maximizing treatment efficacy and minimizing potential side effects. Overall, the advancements in biologics represent a significant leap forward in the EoE market, offering new therapeutic options that address the disease’s underlying causes and improve patient outcomes.
Personalized Medicine: Contributing to Market Expansion
Personalized medicine is transforming the eosinophilic esophagitis market by offering tailored treatment approaches that consider the unique genetic, phenotypic, and clinical characteristics of each patient. This individualized strategy enhances treatment efficacy and minimizes side effects, addressing the variability in patient responses to conventional therapies. One of the key aspects of personalized medicine in EoE is the use of advanced diagnostic tools and biomarkers to better understand the disease’s heterogeneity. By identifying specific biomarkers associated with different EoE subtypes, clinicians can more accurately diagnose and classify the condition. This precise diagnosis enables the selection of targeted therapies that are most likely to be effective for each patient. For example, genetic testing can reveal specific mutations or gene expressions linked to EoE, guiding the choice of treatment. In addition to genetic profiling, personalized medicine in EoE involves detailed phenotypic assessments. This includes evaluating the patient’s clinical history, symptomatology, and response to previous treatments. Through comprehensive assessments, healthcare providers can tailor treatment plans that address individual patient needs. For instance, some patients may benefit more from dietary interventions, while others may respond better to pharmacological treatments such as corticosteroids or biologics.
Moreover, the advent of biologic therapies has significantly contributed to the personalized medicine approach in EoE. Biologics, such as monoclonal antibodies targeting specific cytokines involved in EoE, can be prescribed based on the patient’s unique inflammatory profile. These targeted therapies offer more precise and effective management of the disease, reducing the risk of systemic side effects associated with broad-spectrum treatments. The integration of personalized medicine into EoE management also involves continuous monitoring and adjustment of treatment plans. Advanced diagnostic techniques, such as endoscopic and histological evaluations, allow for real-time assessment of treatment efficacy and disease progression. This dynamic approach ensures that therapy remains aligned with the patient’s evolving condition, optimizing outcomes. Overall, personalized medicine is revolutionizing the EoE market by providing more precise, effective, and patient-centered care.
Enhanced Diagnostic Tools:
Enhanced diagnostic tools are significantly advancing the eosinophilic esophagitis market, providing more accurate and efficient methods for diagnosing and monitoring this chronic condition. The development and implementation of these tools are crucial for early detection, precise disease characterization, and the effective management of EoE. One of the primary advancements in EoE diagnostics is the use of endoscopic techniques combined with advanced imaging technologies. High-resolution endoscopy and endoscopic ultrasound allow for detailed visualization of the esophageal lining, helping to identify characteristic features of EoE such as furrows, rings, and white plaques. These tools facilitate real-time assessment and enable targeted biopsies, improving diagnostic accuracy. Biopsy remains a gold standard in EoE diagnosis, and innovations in histological analysis are enhancing its efficacy. The introduction of minimally invasive techniques, such as the cytosponge, allows for the collection of esophageal cells without the need for traditional endoscopy. This method is less invasive, more comfortable for patients, and can be performed in an outpatient setting, promoting earlier and more frequent monitoring.
Additionally, molecular diagnostics are playing an increasingly important role in the EoE market. Techniques such as quantitative PCR and next-generation sequencing (NGS) enable the detection of specific genetic markers and gene expression profiles associated with EoE. These molecular tools provide insights into the underlying pathophysiology of the disease, allowing for the identification of patient subgroups who may respond differently to various treatments. This precision helps in tailoring personalized therapeutic approaches. Non-invasive biomarkers are also being developed and validated for EoE. Biomarkers found in blood, saliva, or stool samples can indicate the presence of eosinophilic inflammation and monitor disease activity. These biomarkers offer a convenient and less invasive alternative to endoscopy, facilitating regular disease monitoring and timely adjustments to treatment plans. Overall, the advancements in diagnostic tools for EoE are transforming the market by enabling earlier detection, more accurate diagnosis, and personalized treatment strategies.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7588&method=587
Leading Companies in the Eosinophilic Esophagitis Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global eosinophilic esophagitis market, several notable companies are exploring new treatment modalities, biomarkers, and disease mechanisms. This research is driving innovation and potentially leading to the introduction of new therapies into the market. Amgen/AstraZeneca and Ellodi Pharmaceuticals have been investing heavily in their manufacturing capacities in recent months.
Tezepelumab had been designated as an orphan drug (ODD) by the Food and Drug Administration (FDA) in the United States for the treatment of eosinophilic esophagitis. Tezepelumab is being developed by AstraZeneca in conjunction with Amgen and is currently undergoing Priority Review for asthma patients in the United States.
Ellodi Pharmaceuticals stated in May 2024 that Professor Evan Dellon, M.D., M.P.H, would give an oral presentation at the Digestive Disease Week (DDW) 2024 Annual Scientific Meeting. The presentation will emphasize the promising outcomes of APT-1011 (fluticasone propionate orally disintegrating tablet) in the FLUTE-2 Phase 3 clinical trial for eosinophilic esophagitis.
Apart from this, EsoCap AG announced that the ACESO Phase II research comparing ESO-101 to placebo for the treatment of eosinophilic esophagitis achieved its primary aim by significantly lowering the peak eosinophil count in esophageal biopsies.
Request for customization: https://www.imarcgroup.com/request?type=report&id=7588&flag=E
Regional Analysis:
The major markets for eosinophilic esophagitis include the United States, Germany, France, the United Kingdom, Italy, Spain and Japan. According to projections by IMARC, the United States has the largest patient pool for eosinophilic esophagitis while also representing the biggest market for its treatment. This can be attributed to the adoption of targeted therapies that offer more effective treatment options with potentially fewer side effects compared to traditional therapies.
Moreover, there is a growing emphasis on personalized medicine in managing EoE. Advances in genetic and molecular diagnostics are enabling more precise identification of the disease and its subtypes, allowing for tailored treatment approaches. Personalized medicine aims to optimize treatment efficacy and minimize adverse effects by considering individual patient profiles.
Apart from this, there is an increasing focus on developing new pharmacological treatments and dietary interventions. This includes the exploration of novel therapeutic agents and combination therapies to address the diverse needs of EoE patients.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
• United States
• Germany
• France
• United Kingdom
• Italy
• Spain
• Japan
Analysis Covered Across Each Country
• Historical, current, and future epidemiology scenario
• Historical, current, and future performance of the eosinophilic esophagitis market
• Historical, current, and future performance of various therapeutic categories in the market
• Sales of various drugs across the eosinophilic esophagitis market
• Reimbursement scenario in the market
• In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current eosinophilic esophagitis marketed drugs and late-stage pipeline drugs.
In-Market Drugs
• Drug Overview
• Mechanism of Action
• Regulatory Status
• Clinical Trial Results
• Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
• Drug Overview
• Mechanism of Action
• Regulatory Status
• Clinical Trial Results
• Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/eosinophilic-esophagitis-market
IMARC Group Offer Other Reports:
Seborrhea Market: The 7 major seborrhea market is expected to exhibit a CAGR of 7.55% during the forecast period from 2024 to 2034.
Cervix Lesion Market: The 7 major cervix lesion market is expected to exhibit a CAGR of 3.92% during the forecast period from 2024 to 2034.
Extensive-Stage Small Cell Lung Cancer Market: The 7 major extensive-stage small cell lung cancer market is expected to exhibit a CAGR of 7.6% during the forecast period from 2024 to 2034.
Gout Market: The 7 major gout market reached a value of US$ 1.9 Billion in 2023, and projected the 7MM to reach US$ 5.7 Billion by 2034, exhibiting a growth rate (CAGR) of 10.42% during the forecast period from 2024 to 2034.
Growth Hormone Deficiency Market: The 7 major growth hormone deficiency market reached a value of US$ 2.6 Billion in 2023, and projected the 7MM to reach US$ 4.2 Billion by 2034, exhibiting a growth rate (CAGR) of 4.61% during the forecast period from 2024 to 2034.
Melanoma Market: The 7 major melanoma market reached a value of US$ 3.9 Billion in 2023, and projected the 7MM to reach US$ 8.9 Billion by 2034, exhibiting a growth rate (CAGR) of 7.93% during the forecast period from 2024 to 2034.
Neurotrophic Keratitis Market The 7 major neurotrophic keratitis market reached a value of US$ 137.5 Million in 2023, and projected the 7MM to reach US$ 248.0 Million by 2034, exhibiting a growth rate (CAGR) of 5.51% during the forecast period from 2024 to 2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The eosinophilic esophagitis market size reached a value of USD 896.3 Million in 2023. Looking forward, the market is expected to reach USD 7,604.7 Million by 2034, exhibiting a growth rate (CAGR) of 21.46% during 2024-2034. The market is driven by advancements in diagnostic technologies and increased awareness. There is a growing emphasis on personalized treatments, including biologics and novel therapeutic agents. Market growth is also fueled by ongoing research and rising prevalence, with a focus on improving patient outcomes and management strategies.
Advancements in Biologics: Driving the Eosinophilic Esophagitis Market
Recent advancements in biologics are revolutionizing the eosinophilic esophagitis (EoE) market, offering new hope for patients with this chronic and often debilitating condition. Biologics, which are therapeutic agents derived from living organisms, target specific pathways and proteins involved in the inflammatory process of EoE. These treatments have emerged as a significant breakthrough, addressing the limitations of traditional therapies such as corticosteroids and dietary modifications. One of the key advancements is the development of monoclonal antibodies that target specific cytokines involved in the pathogenesis of EoE. For instance, agents like dupilumab, an IL-4/IL-13 inhibitor, have shown promising results in clinical trials by reducing eosinophilic inflammation and improving symptoms. These biologics work by interfering with the immune system's inflammatory responses, thereby providing more targeted and effective treatment options compared to conventional therapies. Another notable advancement is the ongoing research into novel biologics that aim to inhibit eosinophil migration and activation. These therapies focus on the underlying mechanisms driving EoE, such as the overproduction of eosinophils, which are a hallmark of the disease. By addressing these specific pathways, these biologics offer the potential for more durable and sustained relief for patients.
Request a PDF Sample Report: https://www.imarcgroup.com/eosinophilic-esophagitis-market/requestsample
Moreover, the development of these advanced therapies is supported by enhanced diagnostic tools, which allow for more precise identification of patients who are most likely to benefit from biologic treatments. This personalized approach ensures that the right patients receive the most appropriate therapies, maximizing treatment efficacy and minimizing potential side effects. Overall, the advancements in biologics represent a significant leap forward in the EoE market, offering new therapeutic options that address the disease’s underlying causes and improve patient outcomes.
Personalized Medicine: Contributing to Market Expansion
Personalized medicine is transforming the eosinophilic esophagitis market by offering tailored treatment approaches that consider the unique genetic, phenotypic, and clinical characteristics of each patient. This individualized strategy enhances treatment efficacy and minimizes side effects, addressing the variability in patient responses to conventional therapies. One of the key aspects of personalized medicine in EoE is the use of advanced diagnostic tools and biomarkers to better understand the disease’s heterogeneity. By identifying specific biomarkers associated with different EoE subtypes, clinicians can more accurately diagnose and classify the condition. This precise diagnosis enables the selection of targeted therapies that are most likely to be effective for each patient. For example, genetic testing can reveal specific mutations or gene expressions linked to EoE, guiding the choice of treatment. In addition to genetic profiling, personalized medicine in EoE involves detailed phenotypic assessments. This includes evaluating the patient’s clinical history, symptomatology, and response to previous treatments. Through comprehensive assessments, healthcare providers can tailor treatment plans that address individual patient needs. For instance, some patients may benefit more from dietary interventions, while others may respond better to pharmacological treatments such as corticosteroids or biologics.
Moreover, the advent of biologic therapies has significantly contributed to the personalized medicine approach in EoE. Biologics, such as monoclonal antibodies targeting specific cytokines involved in EoE, can be prescribed based on the patient’s unique inflammatory profile. These targeted therapies offer more precise and effective management of the disease, reducing the risk of systemic side effects associated with broad-spectrum treatments. The integration of personalized medicine into EoE management also involves continuous monitoring and adjustment of treatment plans. Advanced diagnostic techniques, such as endoscopic and histological evaluations, allow for real-time assessment of treatment efficacy and disease progression. This dynamic approach ensures that therapy remains aligned with the patient’s evolving condition, optimizing outcomes. Overall, personalized medicine is revolutionizing the EoE market by providing more precise, effective, and patient-centered care.
Enhanced Diagnostic Tools:
Enhanced diagnostic tools are significantly advancing the eosinophilic esophagitis market, providing more accurate and efficient methods for diagnosing and monitoring this chronic condition. The development and implementation of these tools are crucial for early detection, precise disease characterization, and the effective management of EoE. One of the primary advancements in EoE diagnostics is the use of endoscopic techniques combined with advanced imaging technologies. High-resolution endoscopy and endoscopic ultrasound allow for detailed visualization of the esophageal lining, helping to identify characteristic features of EoE such as furrows, rings, and white plaques. These tools facilitate real-time assessment and enable targeted biopsies, improving diagnostic accuracy. Biopsy remains a gold standard in EoE diagnosis, and innovations in histological analysis are enhancing its efficacy. The introduction of minimally invasive techniques, such as the cytosponge, allows for the collection of esophageal cells without the need for traditional endoscopy. This method is less invasive, more comfortable for patients, and can be performed in an outpatient setting, promoting earlier and more frequent monitoring.
Additionally, molecular diagnostics are playing an increasingly important role in the EoE market. Techniques such as quantitative PCR and next-generation sequencing (NGS) enable the detection of specific genetic markers and gene expression profiles associated with EoE. These molecular tools provide insights into the underlying pathophysiology of the disease, allowing for the identification of patient subgroups who may respond differently to various treatments. This precision helps in tailoring personalized therapeutic approaches. Non-invasive biomarkers are also being developed and validated for EoE. Biomarkers found in blood, saliva, or stool samples can indicate the presence of eosinophilic inflammation and monitor disease activity. These biomarkers offer a convenient and less invasive alternative to endoscopy, facilitating regular disease monitoring and timely adjustments to treatment plans. Overall, the advancements in diagnostic tools for EoE are transforming the market by enabling earlier detection, more accurate diagnosis, and personalized treatment strategies.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7588&method=587
Leading Companies in the Eosinophilic Esophagitis Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global eosinophilic esophagitis market, several notable companies are exploring new treatment modalities, biomarkers, and disease mechanisms. This research is driving innovation and potentially leading to the introduction of new therapies into the market. Amgen/AstraZeneca and Ellodi Pharmaceuticals have been investing heavily in their manufacturing capacities in recent months.
Tezepelumab had been designated as an orphan drug (ODD) by the Food and Drug Administration (FDA) in the United States for the treatment of eosinophilic esophagitis. Tezepelumab is being developed by AstraZeneca in conjunction with Amgen and is currently undergoing Priority Review for asthma patients in the United States.
Ellodi Pharmaceuticals stated in May 2024 that Professor Evan Dellon, M.D., M.P.H, would give an oral presentation at the Digestive Disease Week (DDW) 2024 Annual Scientific Meeting. The presentation will emphasize the promising outcomes of APT-1011 (fluticasone propionate orally disintegrating tablet) in the FLUTE-2 Phase 3 clinical trial for eosinophilic esophagitis.
Apart from this, EsoCap AG announced that the ACESO Phase II research comparing ESO-101 to placebo for the treatment of eosinophilic esophagitis achieved its primary aim by significantly lowering the peak eosinophil count in esophageal biopsies.
Request for customization: https://www.imarcgroup.com/request?type=report&id=7588&flag=E
Regional Analysis:
The major markets for eosinophilic esophagitis include the United States, Germany, France, the United Kingdom, Italy, Spain and Japan. According to projections by IMARC, the United States has the largest patient pool for eosinophilic esophagitis while also representing the biggest market for its treatment. This can be attributed to the adoption of targeted therapies that offer more effective treatment options with potentially fewer side effects compared to traditional therapies.
Moreover, there is a growing emphasis on personalized medicine in managing EoE. Advances in genetic and molecular diagnostics are enabling more precise identification of the disease and its subtypes, allowing for tailored treatment approaches. Personalized medicine aims to optimize treatment efficacy and minimize adverse effects by considering individual patient profiles.
Apart from this, there is an increasing focus on developing new pharmacological treatments and dietary interventions. This includes the exploration of novel therapeutic agents and combination therapies to address the diverse needs of EoE patients.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
• United States
• Germany
• France
• United Kingdom
• Italy
• Spain
• Japan
Analysis Covered Across Each Country
• Historical, current, and future epidemiology scenario
• Historical, current, and future performance of the eosinophilic esophagitis market
• Historical, current, and future performance of various therapeutic categories in the market
• Sales of various drugs across the eosinophilic esophagitis market
• Reimbursement scenario in the market
• In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current eosinophilic esophagitis marketed drugs and late-stage pipeline drugs.
In-Market Drugs
• Drug Overview
• Mechanism of Action
• Regulatory Status
• Clinical Trial Results
• Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
• Drug Overview
• Mechanism of Action
• Regulatory Status
• Clinical Trial Results
• Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/eosinophilic-esophagitis-market
IMARC Group Offer Other Reports:
Seborrhea Market: The 7 major seborrhea market is expected to exhibit a CAGR of 7.55% during the forecast period from 2024 to 2034.
Cervix Lesion Market: The 7 major cervix lesion market is expected to exhibit a CAGR of 3.92% during the forecast period from 2024 to 2034.
Extensive-Stage Small Cell Lung Cancer Market: The 7 major extensive-stage small cell lung cancer market is expected to exhibit a CAGR of 7.6% during the forecast period from 2024 to 2034.
Gout Market: The 7 major gout market reached a value of US$ 1.9 Billion in 2023, and projected the 7MM to reach US$ 5.7 Billion by 2034, exhibiting a growth rate (CAGR) of 10.42% during the forecast period from 2024 to 2034.
Growth Hormone Deficiency Market: The 7 major growth hormone deficiency market reached a value of US$ 2.6 Billion in 2023, and projected the 7MM to reach US$ 4.2 Billion by 2034, exhibiting a growth rate (CAGR) of 4.61% during the forecast period from 2024 to 2034.
Melanoma Market: The 7 major melanoma market reached a value of US$ 3.9 Billion in 2023, and projected the 7MM to reach US$ 8.9 Billion by 2034, exhibiting a growth rate (CAGR) of 7.93% during the forecast period from 2024 to 2034.
Neurotrophic Keratitis Market The 7 major neurotrophic keratitis market reached a value of US$ 137.5 Million in 2023, and projected the 7MM to reach US$ 248.0 Million by 2034, exhibiting a growth rate (CAGR) of 5.51% during the forecast period from 2024 to 2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800