Sanofi US
NEWS
Following the FDA’s approval last month, a Centers for Disease Control and Prevention panel has recommended AstraZeneca’s and Sanofi’s Beyfortus for immunizing infants and high-risk young children.
Burjeel Holdings and BridgeBio Pharma ink partnership deal in rare genetic diseases with a plan to set up headquarters in Abu Dhabi.
Despite a challenging economic climate and gloomy forecast, 2023 has still notched some mega-deals for biopharmas. BioSpace highlights the biggest deals in the industry this year.
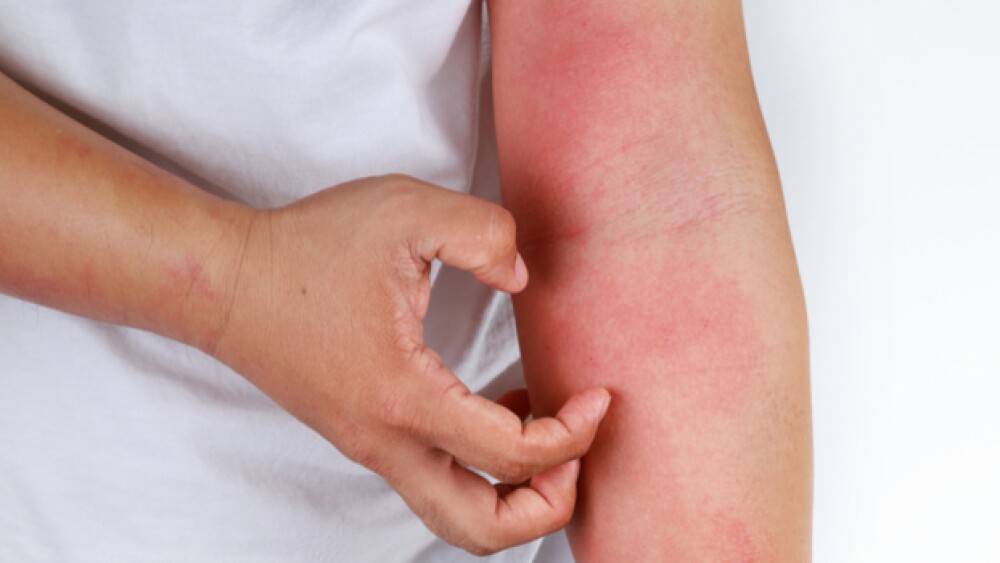
Though the topline data were sparse, Sanofi’s potentially first-in-class candidate amlitelimab improved Eczema Area and Severity Index scores in patients with moderate-to-severe atopic dermatitis.
Following a partial hold on another lead candidate last year, Sanofi is reinvigorating its MS pipeline with a Phase II win for its investigational anti-CD40L antibody frexalimab.
The U.S. Supreme Court unanimously ruled Amgen’s cholesterol drug Repatha patents invalid, ending a protracted legal battle with competitors Sanofi and Regeneron.
As insulin prices skyrocket, pharmas and PBMs faced pointed critique from the Senate HELP Committee, led by Sen. Bernie Sanders (I-VT).
The French pharma drops another BTK inhibitor program from Principia and an anti-TNFa from Ablynx.
Sanofi and Regeneron’s Dupixent (dupilumab) met its primary and all secondary endpoints in the Phase III BOREAS trial, significantly reducing severe exacerbations in COPD.
JOBS
IN THE PRESS