RALEIGH, N.C., May 11, 2015 /PRNewswire/ --
BUNAVAIL® (buprenorphine and naloxone) buccal film showed a 25% average month-over-month growth in prescription sales over the quarter along with significant managed care wins
BELBUCA (buprenorphine HCl) buccal film for treatment of chronic pain PDUFA date of October 23, 2015 associated with $50 million milestone if approved
$10 million milestone payment received from Endo Pharmaceuticals in conjunction with NDA acceptance for BELBUCA
Clonidine Topical Gel Phase 3 data analysis complete; Pathway forward identified
Buprenorphine Depot Injection for the maintenance treatment of opioid dependence and chronic pain on track for year-end 2015 IND filing
ONSOLIS® North American marketing rights re-acquired
First quarter ends with a strong cash position of $63.5 Million
Company to host conference call today at 8:00 AM ET
BioDelivery Sciences International, Inc. (NASDAQ: BDSI) today reported financial results for the first quarter ended March 31, 2015, and reviewed its most significant recent accomplishments and upcoming milestones.
Corporate Update and Recent Accomplishments
- BUNAVAIL (buprenorphine and naloxone) buccal film for the maintenance treatment of opioid dependence
- Nearly 11,000 prescriptions dispensed for BUNAVAIL during Q1 2015; 25% average month-over-month growth in prescription sales over the quarter
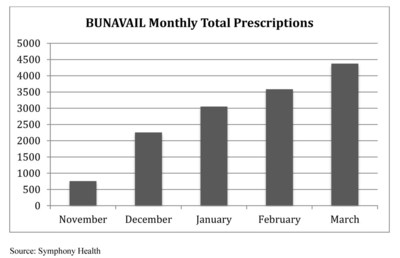
- BUNAVAIL prescriber base expanded to over 2,000
- Market access continues to improve with multiple significant managed care wins in the last 6 weeks including addition to Medicaid formularies in 8 states (Tennessee, Massachusetts, West Virginia, Florida, Maine, Georgia, Nevada, Ohio) representing newly gained access to over 30,000 prescriptions per month
- Recently signed contracts including Express Scripts, OptumRx and CVS/Caremark; BUNAVAIL now available with 3rd tier, unrestricted access in >75% of commercial managed care lives
- Sales force re-targeting initiative completed, aligning sales force activity with high volume prescribers along side the greatest managed market access
- Life cycle management activities underway including initiation of Phase IV clinical experience study and start of a study to support broadening the indication for BUNAVAIL to include induction
- BELBUCA (buprenorphine HCl) buccal film (formerly referred to as BEMA Buprenorphine) for chronic pain
- PDUFA date set for October 23rd of this year
- BDSI received a $10 million milestone payment in first quarter from partner, Endo Pharmaceuticals, an affiliate of Endo International plc (NASDAQ: ENDP), for acceptance of NDA filing; additionally, up to a $50 million milestone payment upon FDA approval
- Phase 3 data being presented at the upcoming American Pain Society 2015 Annual Scientific Meeting on May 14
- Clonidine Topical Gel for painful diabetic neuropathy
- Primary endpoint in phase 3 clinical study not met (n=260; p= 0.45); several secondary efficacy endpoints statistically significant; positive safety profile
- Data analysis determined that key inclusion criteria of placebo period responsiveness and response to capsaicin were too broad and led to failed outcome.
- Final study analysis identified a sizeable patient population (capsaicin score >4; <1 point change in placebo run-in) with statistically significant (n=158; p<0.02) improvement in pain score versus placebo)
- Next steps being considered include a potential smaller scale study utilizing an adaptive study design with the noted improvements in the placebo and capsaicin inclusion criteria; study start not anticipated prior to 4th quarter
- Buprenorphine Depot Injection for the maintenance treatment of opioid dependence and chronic pain
- Formulation development work initiated early in quarter; anticipated IND filing by year-end for a formulation potentially capable of providing 30 days of continuous therapy
- ONSOLIS® (fentanyl buccal soluble film) for management of breakthrough pain in opioid tolerant patients with cancer
- North American rights reacquired from Meda; Meda continues marketing and sales efforts in the E.U.
- Reformulation data submitted to FDA during first quarter to allow ONSOLIS to return to U.S. marketplace; 6 month review underway
- Continue to consider commercialization options
"We continue to make progress with the launch of BUNAVAIL," said Dr. Mark A. Sirgo, President & CEO of BioDelivery Sciences. "In particular, we are making significant advancements in securing managed care and pharmacy access to BUNAVAIL, having recently made headway with a number of important managed care plans and addition to eight state Medicaid plans, including Tennessee and Massachusetts, providing additional access to over 30,000 prescriptions each month. We believe these achievements will be critical to generating meaningful pull-through from our BUNAVAIL launch efforts as we move through this quarter, and particularly, as we progress through the second half of this year."
Dr. Sirgo continued, "In addition, we look forward to the October 23rd PDUFA date for BELBUCA, and the upcoming presentation of Phase 3 pivotal trial data at the Annual Meeting of the American Pain Society. Also, following our completion of the analysis of the first Phase 3 study for Clonidine Topical Gel, we determined that too lenient placebo response and capsaicin score entry criteria led to the study failure. Based on these findings we remain optimistic in the potential of the product as a possible treatment for painful diabetic neuropathy, and continue to evaluate the appropriate strategy for this product candidate going forward. Finally, we continue to make progress with formulation development work for Buprenorphine Depot in order to identify the appropriate formulation to advance into the clinic, and anticipate filing an IND for this product candidate by the end of the year with human pharmacokinetic studies beginning in the first quarter of next year."
First Quarter 2015 Financial Results Overview
- Net revenue for the first quarter ended March 31, 2015, was $13.1 million, compared to $20.7 million in the corresponding period of 2014
- Total operating expenses for the first quarter ended March 31, 2015, were $19.7 million, compared to $19.3 million in the corresponding period of 2014
- Net loss for the first quarter ended March 31, 2015, was $8.2 million, or $0.16 per diluted share, compared to $4.6 million, or $0.11 per diluted share, in the corresponding period of 2014
- As of March 31, 2015, BDSI had $63.5 million in cash and cash equivalents, as compared to $70.5 million as of December 31, 2014
Key Anticipated 2015 Milestones
- Anticipated approval of BELBUCA (October 23 PDUFA date) and an associated milestone payment from Endo of up to $50 million
- Completion of an induction study for BUNAVAIL and sNDA submission by end of year
- Approval of a data package by the end of 3Q 2015 in order to reintroduce ONSOLIS in 2016
- Filing of an Investigational New Drug (IND) application for Buprenorphine Depot by end of year
Conference Call & Webcast
Monday, May 11, 2015 @ 8am Eastern/5:00am Pacific | |
Domestic: | 888-359-3627 |
International: | 719-325-2323 |
Conference ID: | 7599006 |
Webcast: | |
Replays available until May 25, 2015 | |
Domestic: | 877-870-5176 |
International: | 858-384-5517 |
Conference ID: | 7599006 |
About BioDelivery Sciences International
BioDelivery Sciences International, Inc. is a specialty pharmaceutical company with a focus in the areas of pain management and addiction medicine. BDSI is utilizing its novel and proprietary BioErodible MucoAdhesive (BEMA®) technology and other drug delivery technologies to develop and commercialize, either on its own or in partnership with third parties, new applications of proven therapies aimed at addressing important unmet medical needs.
BDSI's development strategy focuses on utilization of the FDA's 505(b)(2) approval process. This regulatory pathway creates the potential for more timely and efficient approval of new formulations of previously approved therapeutics.
BDSI's particular area of focus is the development and commercialization of products in the areas of pain management and addiction. These are areas where BDSI believes its drug delivery technologies and products can best be applied to address critical unmet medical needs. BDSI's marketed products and those in development address serious and debilitating conditions such as breakthrough cancer pain, chronic pain, painful diabetic neuropathy and opioid dependence.
For more information, please visit or follow us: | |
Internet: | |
Facebook: | Facebook.com/BioDeliverySI |
Twitter: | @BioDeliverySI |
About BUNAVAIL
INDICATION
BUNAVAIL (buprenorphine and naloxone) Buccal Film (CIII) is a prescription medicine indicated for the maintenance treatment of opioid dependence. BUNAVAIL should be used as part of a complete treatment plan to include counseling and psychosocial support.
Prescription use of this product is limited under the Drug Addiction Treatment Act (DATA).
IMPORTANT SAFETY INFORMATION
Keep BUNAVAIL (buprenorphine and naloxone) Buccal Film (CIII) out of the sight and reach of children. Ingestion of BUNAVAIL by a child may cause severe breathing problems and death. If a child takes BUNAVAIL, get emergency help right away.
Do not take BUNAVAIL if you are allergic to buprenorphine or naloxone, as serious negative effects including anaphylactic shock, have been reported.
Do not take BUNAVAIL before the effects of other opioids (e.g., heroin, methadone, oxycodone, morphine) have lessened as you may experience withdrawal symptoms.
Do not drive, operate heavy machinery, or perform any other dangerous activities until you know how BUNAVAIL affects you.
BUNAVAIL contains buprenorphine, an opioid that can cause physical dependence. Your doctor can tell you more about the difference between physical dependence and drug addiction. Do not stop taking BUNAVAIL without talking to your doctor. You could become sick with uncomfortable withdrawal symptoms because your body has become used to this medicine.
Do not switch from BUNAVAIL to other medicines that contain buprenorphine without talking with your doctor. The amount of buprenorphine in a dose of BUNAVAIL is not the same as the amount of buprenorphine in other medicines. Your doctor will prescribe a dose of BUNAVAIL that may be different than other buprenorphine-containing medicines you may have been taking.
BUNAVAIL can cause serious life-threatening breathing problems, overdose and death, particularly when taken by the intravenous (IV) route in combination with benzodiazepines, sedatives, tranquilizers or alcohol. You should not drink alcohol while taking BUNAVAIL, as this can lead to loss of consciousness or even death.
Like other opioids (e.g., heroin, methadone, oxycodone, morphine), BUNAVAIL may produce orthostatic hypotension ('dizzy spells') in ambulatory individuals.
Common side effects of BUNAVAIL include headache, drug withdrawal syndrome, lethargy (lack of energy), sweating, constipation, decrease in sleep (insomnia), fatigue and sleepiness.
Because BUNAVAIL contains naloxone, injecting BUNAVAIL may cause serious withdrawal symptoms such as pain, cramps, vomiting, diarrhea, anxiety, sleep problems, and cravings.
BUNAVAIL can be abused in a manner similar to other opioids, legal or illicit. Keep BUNAVAIL in a safe place. Do not give your BUNAVAIL to other people, it can cause them harm or even death. Selling or giving away this medicine is against the law.
BUNAVAIL is not recommended in patients with severe hepatic impairment. BUNAVAIL may be used with caution for maintenance treatment in patients with moderate hepatic impairment.
Before taking BUNAVAIL, tell your doctor if you are pregnant or plan to become pregnant. If you become pregnant while taking BUNAVAIL, tell your doctor immediately as there may be significant risks to you and your baby; your baby may have symptoms of withdrawal at birth.
Before taking BUNAVAIL, talk to your doctor if you are breastfeeding or plan to breastfeed your baby. BUNAVAIL can pass into your breast milk and may harm your baby. Monitor your baby for increased sleepiness and breathing problems. Your doctor should tell you about the best way to feed your baby if you are taking BUNAVAIL.
To read full press release, please click here.
Help employers find you! Check out all the jobs and post your resume.




