SARASOTA, Fla., Oct. 9, 2014 /PRNewswire-USNewswire/ -- RPS Diagnostics, Inc. (RPS®) today announced CE mark of its FebriDx test, clearing the way for sales in the European Union and all countries recognizing the CE mark.
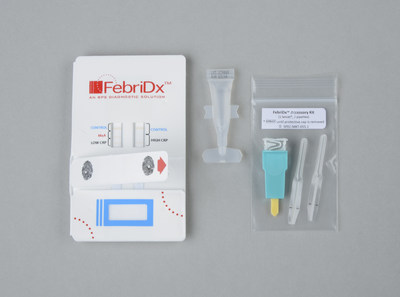
FebriDx is a rapid, disposable, in-office test that helps identify and differentiate a clinically significant immune response to viral and/or bacterial acute febrile respiratory infection at the point-of-care. The FebriDx test combines the interpretation of both Myxovirus Resistance Protein A (MxA) an interferon derivative that becomes elevated in the presence of acute viral infection, and C-reactive Protein (CRP) an acute-phase protein that is elevated in the presence of bacterial infection. This novel and proprietary pattern of results provides an accurate way to identify patients suffering from a clinically significant viral and/or bacterial acute febrile respiratory infection from those patients with a microbiologically unconfirmed respiratory illness (MURI).
Using only a fingerstick blood sample, the FebriDx test can be performed by a nurse or technician during a patient's initial workup. Results are available for the clinician in as soon as ten minutes, allowing a treatment plan to be established during the patient's initial visit.
"Obtaining the FebriDx CE mark is a significant milestone for RPS," said Robert Sambursky, MD, chief executive officer and president of RPS. "The FebriDx test will advance our goal to positively impact global healthcare by facilitating an earlier diagnosis, helping to reduce the spread of disease and encourage the judicious use of antibiotics."
Acute febrile respiratory infections represent a major source of morbidity, mortality, and healthcare costs. Identifying a clinically significant respiratory infection and differentiating viral from bacterial etiology during the office visit is challenging because of overlapping symptoms and signs. According to Rattinger et al, more than half of all antibiotics are prescribed to outpatients with acute respiratory infection; many of these prescriptions are not needed and lead to avoidable adverse reactions and antibiotic resistance.
Pending distribution arrangements, the FebriDx test will soon be commercially available in Europe. The test has not yet been submitted for U.S. Food and Drug Administration (FDA) review. To subscribe to future FebriDx product updates and announcements, please email febridx@rpsdetectors.com.
About RPS
Founded in 2004, RPS Diagnostics, Inc. (RPS) is an emerging developer, manufacturer, and marketer of rapid point-of-care (POC) diagnostic tests. The company's innovative and patented technology platform facilitates the development of a spectrum of cost-effective tests to support the rapid diagnosis of patients with infectious diseases and inflammatory conditions. As a result of U.S. government contracts, this platform is also being developed to help detect the body's immune response to viral and bacterial infections as well as chemical nerve agent blood toxins. RPS tests have high sensitivity and specificity, and can be easily performed by a clinician or their staff without extensive training or additional equipment. Currently available RPS tests include AdenoPlus® to aid in the diagnosis of Adenoviral conjunctivitis (pink eye) and InflammaDry® to aid in the diagnosis of dry eye disease. For more information on RPS or its products, visit RPSdetectors.com.
Media contact: Laura Lovejoy laura@saranova.net 941.928.9025
Photo - http://photos.prnewswire.com/prnh/20141008/151092
SOURCE RPS Diagnostics, Inc. (RPS)
