Regulatory
Despite a patent extension, Merck’s muscle relaxant reversal injection is now facing potential generic competition from Hikma Pharmaceuticals, which is seeking the FDA’s approval for a copycat version.
Johnson & Johnson and Legend Biotech got a positive opinion from a European Medicines Agency panel for earlier lines of treatment, as they ready for a March FDA advisory committee meeting.
The FDA’s target decision date is June 27, 2024, the companies announced Friday. Sanofi and Regeneron are looking to expand blockbuster Dupixent into chronic obstructive pulmonary disease.
The litigation alleges the regulator allowed its competitor Liquidia to skirt FDA precedents by amending an already pending NDA to add a PH-ILD indication instead of filing a new application.
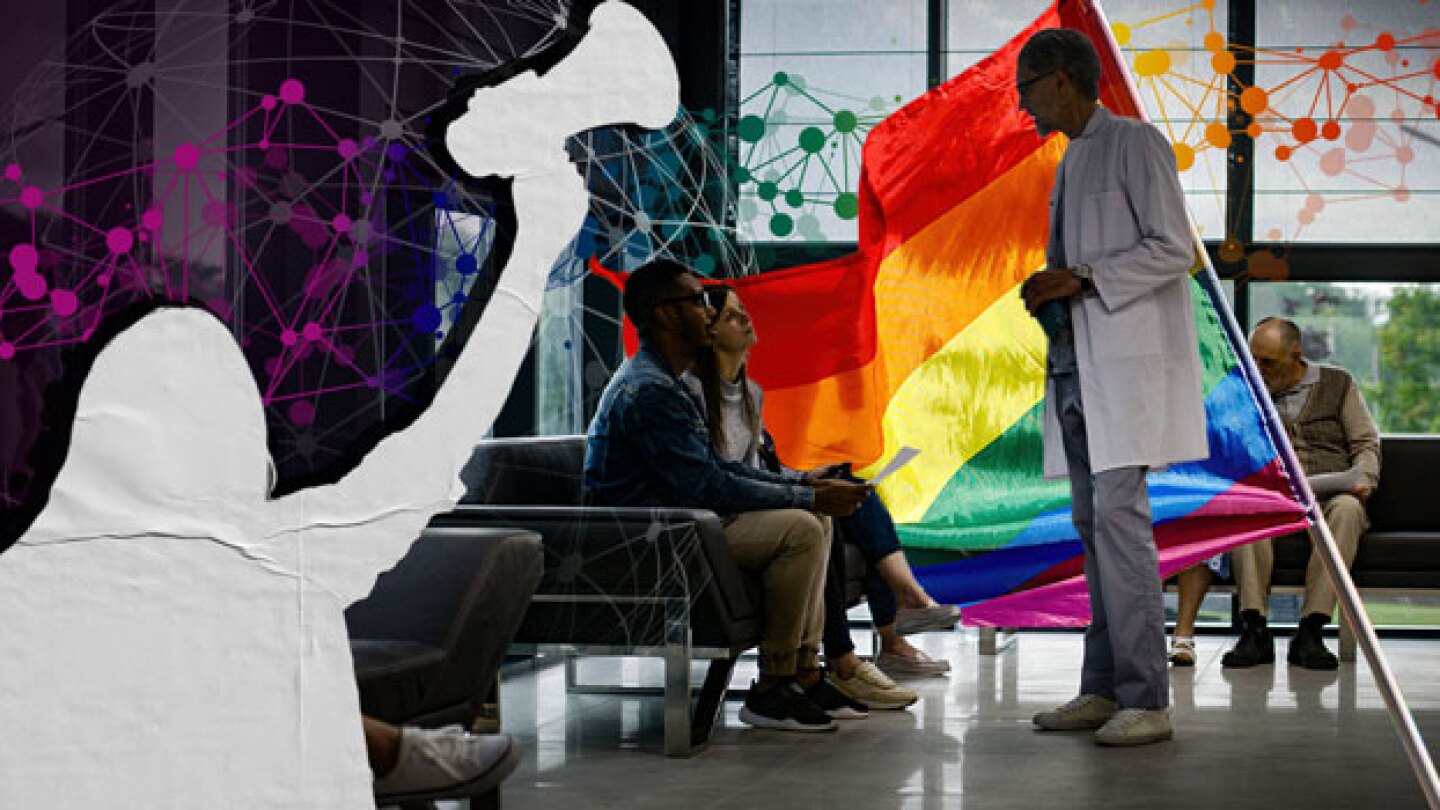
With over 20% of people born between 1997 and 2003 identifying as a sexual and gender minority, moderated panels at SCOPE 2024 discuss the need to engage this community in clinical trials.
Mergers and acquisitions are trending upward as Novo Nordisk, Gilead, and Johnson & Johnson kick off the year with big deals. AI and other scientific advances will likely be the focus of M&As yet to come.
The regulator has placed a clinical hold on RAPT Therapeutics’ drug zelnecirnon, which was being investigated in atopic dermatitis and asthma, after a patient experienced liver failure.
The European Commission granted marketing authorization in the EU to treat patients 16 years of age and older with moderately to severely active ulcerative colitis.
A week after Britain’s debut of the four-week Kwikpen, a European Medicines Agency panel is slated to review Eli Lilly’s multi-dose, pre-filled pen injector for diabetes drug Mounjaro.
The companies’ Biologics License Application for antibody-drug conjugate datopotamab deruxtecan has been accepted by the FDA for locally advanced or metastatic nonsquamous non-small cell lung cancer.
PRESS RELEASES